
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
What is soluble, insoluble, slightly soluble and Solubility-product constant and Effect of pH on Solubility?
Solubility Product
Saturated solution contains maximum amount of solute possible at given temp with an undissolved excess of substance
Not necessarily concentrated
Depends on solubility of solute General rule used to express solubility's
Substance is said to be soluble if solubility is greater than 1 g per 100 g of water
Insoluble à less than 0.1 g in 100 g water
Slightly soluble à between 0.1-1.0 g per 100 g water
Solubility-product constant à of a substance the product of molar concentrations of its ions in a saturated solution, each raised to the power that is the coefficient of that ion in the chemical equation
Effect of pH on Solubility
d) Sometimes it is necessary to account for other reactions aqueous ions might undergo.
For example, if the anion is the conjugate base of a weak acid, it will react with H3O+.
You should expect the solubility to be affected by pH.
Consider the following equilibrium.
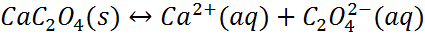
Because the oxalate ion is conjugate to a weak acid (HC2O4-), it will react with H3O+.
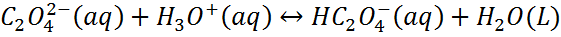
According to Le Chateliers principle, as C2O42- ion is removed by the reaction with H3O+, more calcium oxalate dissolves.
What is Complex-Ion, how to express formation constant of complexation equilibrium and Complex-Ion Dissociation canstant? What is Oxidation-reduction reactions?
u A complex ion is an ion formed from a metal ion with a Lewis base attached to it by a coordinate covalent bond.
A complex is defined as a compound containing complex ions.
A ligand is a Lewis base (an electron pair donor) that bonds to a metal ion to form a complex ion.
u The aqueous silver ion forms a complex ion with ammonia in steps.
Ag (aq) NH3 (aq)  Ag(NH3 ) (aq)
Ag(NH3 ) (aq)
Ag(NH3 ) (aq) NH3 (aq)  Ag(NH3 )2 (aq)
Ag(NH3 )2 (aq)
When you add these equations, you get the overall equation for the formation of Ag(NH3)2+.
Ag (aq) 2NH3 (aq)  Ag(NH3 )2 (aq)
Ag(NH3 )2 (aq)
u The formation constant of complexation equilibrium, Kf, is the equilibrium constant for the formation of a complex ion from the aqueous metal ion and the ligands.
Ag (aq) 2NH3 (aq)  Ag(NH3 )2 (aq)
Ag(NH3 )2 (aq)
The formation constant for Ag(NH3)2+ is:
| The value of K | f for Ag(NH3 )2 + is 1.7 x 107. | ||||
The large value means that the complex ion is quite stable.
When a large amount of NH3 is added to a solution of Ag+, you expect most of the Ag+ ion to react to form the complex ion.
Complex-Ion Dissociation
u The dissociation constant, Kd, is the reciprocal, or inverse, value of Kf.
The equation for the dissociation of Ag(NH3)2+ is
| (aq) | Ag | (aq) 2NH3 | (aq) | |||||||
| Ag(NH3 )2 | ||||||||||
| The equilibrium constant equation is | ||||||||||
| Kd | [Ag ][NH3 ]2 | |||||||||
| Kf | ] | |||||||||
| [Ag(NH3 )2 |
 Dissociation equilibrium and stability constant:
Dissociation equilibrium and stability constant:
E.g., formation of CaY2- : Ca2+ + Y4- = CaY2-
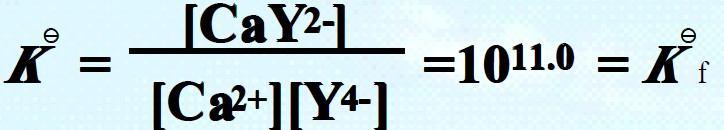
Comparing with that of ZnY2-, Kf = 1016.4 .
The magnitude of Kf signifies the stability of the complex as well as the completeness of the complexation reaction. The dissociation constant Kd will be the reciprocal of the formation constant, which signifies the instability of a complex.
 Formation of [Cu(NH3)4]2+
Formation of [Cu(NH3)4]2+
Cu2+ + NH3 = [Cu(NH3)]2+
[Cu(NH3)]2+ + NH3 = [Cu(NH3)2]2+
[Cu(NH3)2]2+ + NH3 = [Cu(NH3)3]2+
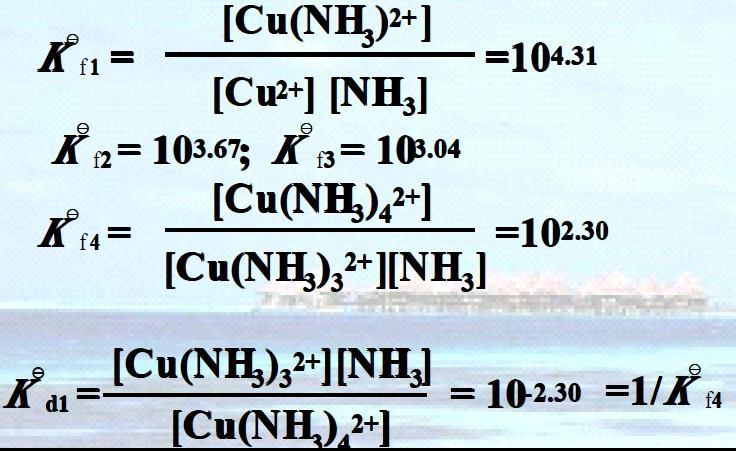 [Cu(NH3)3]2+ + NH3 = [Cu(NH3)4]2+
[Cu(NH3)3]2+ + NH3 = [Cu(NH3)4]2+
In general Kf 1 > Kf 2 > Kf 3 > Kf 4
i.e., the stepwise formation constant will decrease with the increase of coordination number.
Oxidation-reduction reactions are electron transfer reactions
The process may involve the complete transfer of electrons to form ionic bonds or only a partial transfer or shift of electrons to form covalent bonds.

Oxidation and reduction occur simultaneously in a chemical reaction; one cannot take place without the other.
Oxidation
is the loss of electrons by a particle in a reaction, resulting in an increase in the oxidation number.
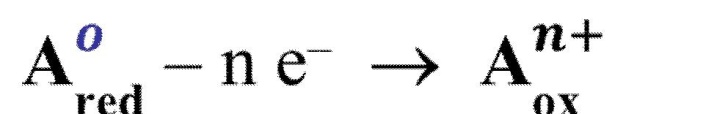
Reduction
is the gain of electrons by a particle in a reaction that results in a decrease in the oxidation number. 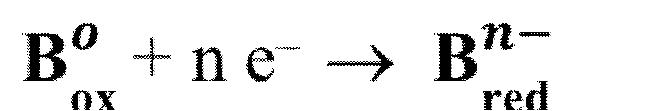
Factors affecting the complex equilibrium, Which rules help in assessing oxidation numbers?
Factors affecting the complex equilibrium
Ligands concentrations pH of solutions
Ionic strength of solution
1. Influence of Components or ligands concentration.
If Components or ligands concentrations are high, the complex ions of coordinate number is high.
Mz+ + L = MLz+
MLz+ + L = ML2z+
ML2z+ + L = ML3z+
2. Influence of pH
If ligand is salt of strong acid, pH does not influence to complexation
process, b/c ionization of strong acids are completely. The
concentration of hydrogen changes just ionic strength.
If ligand is salt of weak acid, pH influences to ionization of ligands, that
time additional process occurs.
If adding more hydrogen concentration, the equilibrium process is going right side, and influence to destroyed comlpexation.
3. Influence ionic strength of solutions
Change ionic strength to influence ions active coefficient changes, that influence complexation equilibrium process.
Rules that help in assessing oxidation numbers:
The oxidation number of any free element is zero, even when the atoms are combined with themselves (e.g. O2, P4, S8).
No regard is paid to covalent bonds between atoms of the same species.
An element may have more than one oxidation number, if it forms a variety of compounds.
The oxidation number of hydrogen in a compound or an ion is + I except in ionic hydrides ( I).
The oxidation number of oxygen in a compound or in an ion is II except in peroxides (it takes on a I).
Metals generally have only positive oxidation numbers in compounds.
The oxidation number of alkali metals equals always + I,
of alkaline earth metals always + II.
Nonmetals have negative oxidation numbers when combined with metals, positive oxidation numbers when combined with more electronegative nonmetals.
Date: 2015-01-29; view: 1960
| <== previous page | | | next page ==> |
| Calculating the pH of an Aqueous Solution of the Salt of a Weak Acid and weak base? | | | What is oxidant and reductant? What is the half-reactions and redox pairs? |