
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
A New Oxocarbon C12O6 via Highly Strained Benzyne Intermediates
In the article new types of oxonium ions, such as 1-3 bold, minus were discuss. Their reactionary ability and stability was compared to a well-known oxaadamantane (4). Also resulted them some descriptions (for example, bond lengths and bond angles), described as succeeded to distinguish so eccentric structures and how they were investigated.. Their importance is also reasonable, as electrophilic alkylatings agents. There are a few ways how to take oxonium ions that were distinguished before (4) not clear and also those which was investigated first (1-3) bold, minus in this article. not clear
Graphical abstract
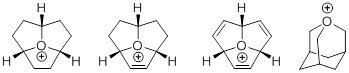
1 2 3 4
A New Oxocarbon C12O6 via Highly Strained Benzyne Intermediates
Abstract
Elimination of tosilate from what? followed by [2+2]cycloaddition with ketene silyl acetals facilitates the formation of highly strained benzynes with containing annulated cyclobutane rings. Such compounds deserve attention with respect to molecular strain and the possible bond-length alternation. They can be applicated applied for in multidirectional ring-expansion reactions merge. According to this method here was shown the synthesis of the novel oxocarbon C12O6.
Graphical Abstract Реклама
Date: 2014-12-22; view: 1224
| <== previous page | | | next page ==> |
| Abstract | | | Abstract align, comment |