
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Abstract align, comment
Abstract
Elimination of tosilate from what? followed by [2+2]cycloaddition with ketene silyl acetals facilitates the formation of highly strained benzynes with containing annulated cyclobutane rings. Such compounds deserve attention with respect to molecular strain and the possible bond-length alternation. They can be applicated applied for in multidirectional ring-expansion reactions merge. According to this method here was shown the synthesis of the novel oxocarbon C12O6.
Graphical Abstract Ðåêëàìà
Abstract align, comment
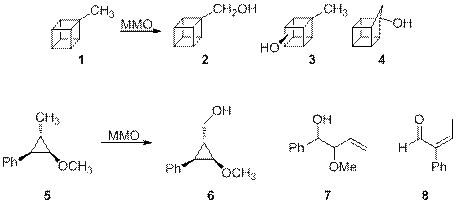
The mechanisms of catalytic hydroxylation of unactivated C-H bonds by MMOH what is this? enzymes are not fully understood, however, and remain a subject of considerable research effort. Change-remove
The accumulated accumulated where? results of probe studies of MMO hydroxylations permit the firm conclusion that no radical intermediates are formed.
The rearrangement product from sMMO-catalyzed what is this? hydroxylation of methylcubane (1) is 1-homocubanol (4), formed via a cationic rearrangement, and production of a cationic species was also demonstrated in sMMO hydroxylation of probe 5. Rasshirit’
Date: 2014-12-22; view: 1259