
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Abstract
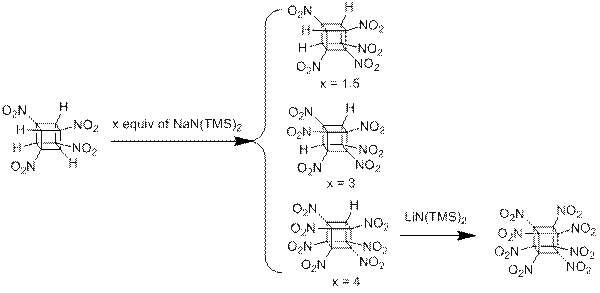
Successful methodology for the synthesis of highly nitrated cubanes was developed. Four of the eight nitro groups of octanitrocubane 1 are introduced by dimethyldioxirane oxidation of the
tetraamine derived from Curtius-type transformations of the corresponding tetraacid; three more by the astonishingly rapid, low-temperature N2O4 nitration of sequentially formed polynitrocubyl anions via 4 equiv of NaN(TMS)2 at -78.8°C; and last by addition of excess nitrosyl chloride to a solution of the lithium salt of heptanitrocubane (from 4 and LiN(TMS)2) in CH2Cl2 at -78.8°C followed by ozonation. Application of the Kamlett-Jacobs equations to octanitrocubane leads to calculated detonation velocities and pressures much higher than classic C-nitro explosive TNT, N-nitro compound HMX, and polycyclic nitramine CL-20.
Abstract
In the article new types of oxonium ions, such as 1-3 bold, minus were discuss. Their reactionary ability and stability was compared to a well-known oxaadamantane (4). Also resulted them some descriptions (for example, bond lengths and bond angles), described as succeeded to distinguish so eccentric structures and how they were investigated.. Their importance is also reasonable, as electrophilic alkylatings agents. There are a few ways how to take oxonium ions that were distinguished before (4) not clear and also those which was investigated first (1-3) bold, minus in this article. not clear
Graphical abstract
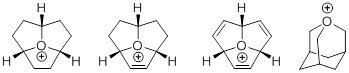
1 2 3 4
Date: 2014-12-22; view: 1219
| <== previous page | | | next page ==> |
| Abstract Mnogo lishnego! | | | A New Oxocarbon C12O6 via Highly Strained Benzyne Intermediates |