
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Figure 10. Formation of Hydrogen Bonds between water molecules
Thus when water molecules are close together, their positive and negative regions are attracted to the oppositely-charged regions of nearby molecules. The force of attraction, shown here as a dotted line, is a hydrogen bond. The δ+ Hydrogen is so strongly attracted to the electron pair that it is almost as if you were beginning to form a co-ordinate (donor-acceptor covalent) bond. Notice that each water molecule can potentially form four Hydrogen bonds. It doesn't go that far, but the attraction is significantly stronger than an ordinary interaction. Hydrogen bonds have about a tenth of the strength of an average covalent bond, and are being constantly broken and reformed in liquid water.
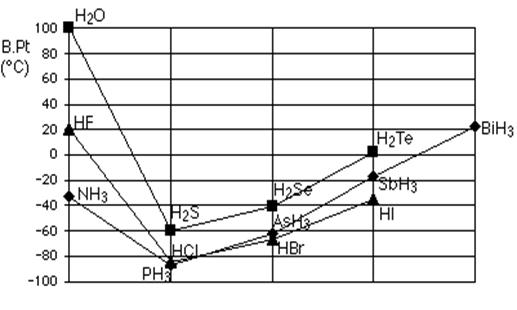
This is why the boiling point of water is higher than that of ammonia or Hydrogen Fluoride (see Fig. 11). In the case of Ammonia, the amount of hydrogen bonding is limited by the fact that each Nitrogen only has one electron pair. In a group of Ammonia molecules, there aren't enough electron pairs to go around to satisfy all the Hydrogens. In Hydrogen fluoride, the problem is a shortage of hydrogens. In water, there are exactly the right number of each. Water could be considered as the "perfect" Hydrogen bonded system.
The hydrogen bonds that form between water molecules account for some of the essential and unique properties of water:
Ø The attraction created by hydrogen bonds keeps water liquid over a wider range of temperature than is found for any other molecule its size.
Ø The energy required to break multiple hydrogen bonds causes water to have a high heat of vaporization; that is, a large amount of energy is needed to convert liquid water, where the molecules are attracted through their hydrogen bonds, to water vapor, where they are not.
Two outcomes of this:
Date: 2015-01-12; view: 1506