
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
PRACTICE PROBLEMS
1. Without looking at the Periodic Table, give the group, period, and block in which the element that has the following electron configuration, is located: [Xe]6s2.
2. Without looking at the Periodic Table, write the electron configuration for the element in the third period in Group 1. Is this likely to be more or less active than that described in question #1?
3. Without looking at the Periodic Table, give the group, period, and block in which the element with the following shorthand electron configuration is located: [Kr]5s1.
4. How does the reactivity of the element in question # 1 compare with that in Group 1 of the same group?
CHAPTER # 4. CHEMICAL BONDING
Sourses:
1. Introduction in General, Organic and Biochemistry, 7th Edition, by Morris Hein, Leo R. Best, Scott Pattison and Susan Arena, Brooks/Cole Publishing Co., 2001. (Chapter 6, pp. 161-190);
2. http://hyperphisics.phy-astr.gsu.edu/hbase/chemical/bond.html
 Isolated and electrically neutral atoms are rare in nature. Ordinarily, only noble-gas atoms exist independently. Atoms of other elements are usually combined with each other or with atoms of different elements. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms (Fig. 6).
Isolated and electrically neutral atoms are rare in nature. Ordinarily, only noble-gas atoms exist independently. Atoms of other elements are usually combined with each other or with atoms of different elements. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms (Fig. 6).

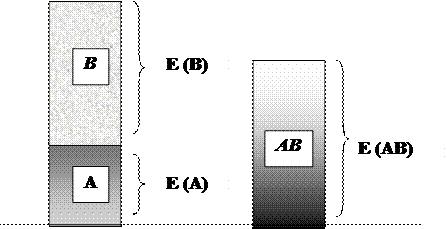 |
∑ (E(A) + E(B)) > E (AB)
A, B - separated atoms;
AB - compound
Figure 6. Condition for chemical bond formation
Atoms are held together by electrostatic attraction between positively charged nuclei and negatively charged electrons. This attraction permits two atoms to be held together by a chemical bond, which is a link between atoms that results from the mutual attraction of their nuclei for electrons. Chemical bonds are classified by the way in which valency electrons are distributed around the nuclei of the combined atoms.
1. Types of Chemical Bonds
In previous chapter we described how atoms of main group elements could gain or lose electrons to form ions with noble-gas electron configurations. Metals tend to lose electrons to form positive ions, and non-metals tend to gain electrons to form negative ions. Many chemical compounds are composed of ions; in these compounds the chemical bond is an ionic one. An ionic bond is the chemical bond resulting from electrostatic attraction between positive and negative ions. If these is a purely ionic bond, one atom has completely given up one or more electrons, and another atom has gained them - as illustrated for two atoms that each have one unpaired electron at the top in Figure 7.
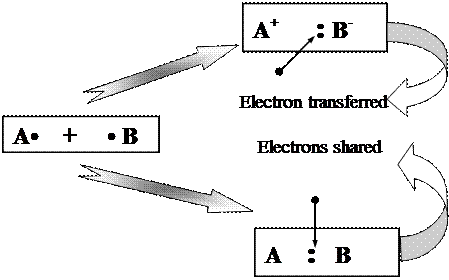 | |||
| |||
| |||
Figure 7. Two main types of chemical bond formation (in ionic bond, electrons are transferred from one atom to another, and positive and negative ions are formed. In covalent bond, an electron pair is shared between two atoms)
In the second major type of chemical bond, called covalent bond, neither bonding atom completely loses or gains an electron or electrons. A covalent bond is a chemical bond resulting from the sharing of electrons between two atoms. A covalent bond in which two electrons are shared is represented by a pair of electron dots, as shown at the bottom right-hand corner in Figure 7. In a purely covalent bond, the shared electrons are “owned” equally by the two atoms.
Chemical bonds between unlike atoms are never completely ionic and rarely completely covalent. Bonds can be anywhere in the range between the bonded atoms attract electrons.
The degree to which bonds are ionic or covalent can be estimated by comparing of electronegativity of the bonded atoms. The more two atoms differ in electronegativity, the more ionic the bond is between them. In other words, the electrons spend more time close to the bonded atom that attracts them more strongly and hence cause that atom partially resemble an anion and the other atom, a cation.
Figure 8 can be used to classify bonds according to electronegativity differences. The electronegativity (see Appendix 7) of one bonded atom is subtracted from that of the other. For example, the electronegativity difference between a Cesium (Cs) atom and a Fluorine (F) atom is 4,0 - 0,7 = 3,3. According to Figure 8, a Cesium-Fluorine bond is an ionic one. In fact it is one of the most highly ionic bonds known.
| % Ionic character | 100% |
| 5 % |
| ||||
| Difference in electro-negativity | 4,0 | 1,7 | 0,3 | 0,0 | ||||

|  
| |||||||
 Type of bond Type of bond
| 
| 
| ||||||
| Ionic | Polar covalent | Nonpolar covalent | ||||||
Figure 8. The variation of bond type with percent ionic character and the electronegativity difference between the bonded atoms
Bonds that have an ionic character of 50% or less are classified as covalent bonds. A bond between identical atoms is completely covalent. Hydrogen, for example, exists in nature not as isolated atoms, but as pairs of atoms held together by covalent bonds, H:H. The Hydrogen-Hydrogen bond has 0% ionic character. It is nonpolar-covalent bond, a covalent bond in which the bonding electrons are shared equally by the bonded atoms, with a resulting balanced distribution of electrical charge. Bonds having 0%-5% ionic character, corresponding to electronegativity differences of roughly 0 to 0,3, are generally considered as nonpolar covalent bonds. For example, because the electronegativity difference between Hydrogen (H) and Boron (B) is 0,1, they form a bond that is essentially nonpolar.
In bonds with significantly different electronegativities, the electrons are more attracted to the more electronegative atom. Such bonds are polar, meaning that they have an uneven distribution of charge. Covalent bonds having 5%-50% ionic character are classified as polar. A polar-covalent bond is a covalent bond in which the united atoms have an unequal attraction for the shared electrons.
Nonpolar and polar-covalent bonds are compared in the sketches in Figure 9 of the electron density in Hydrogen-Hydrogen and Hydrogen-Chlorine bonds. Hydrogen and Chlorine atoms combine to produce the compound known as Hydrogen Chloride (HCl). The electronegativity difference between Chlorine and Hydrogen atoms is 3,0 - 2,1 = 0,9, indicating formation of a polar-covalent bond. The electrons in this bond spend more of their time near more electronegative Chlorine atom than near the Hydrogen atom, as indicated in Figure 9b. Consequently, the Chlorine end of the bond has a relative surplus of electrons and a partial negative charge, indicated by writing δ-. The Hydrogen end of the bond then has an equal partial positive charge δ+.
 |
a) Nonpolar-covalent bond



b)  Polar-covalent bond
Polar-covalent bond
δ+ δ-
Figure 9. Comparison of the electron density in (a) a non-polar, Hydrogen-Hydrogen bond and (b) polar Hydrogen-Chlorine bond
In addition to ionic and covalent bond, there is a third major type of bond - metallic bond. In solid or liquid metals, metal atoms give up electrons, as in ionic compounds. The liberated electrons however, are free to move throughout the material, rather than being held in place in negative ions.
In general, atoms of non-metals form covalent bonds with each other, atoms of metals form metallic bonds with each other, and atoms of metals form ionic bonds with atoms of non-metals. There are many exceptions, however. One common and important exception is the formation of polar-covalent bonds between metals and non-metals that do not differ greatly in electronegativity.
Sample.
Use electronegativity differences and Figure 8, to classify bonds between Sulfur and the following elements: Hydrogen, Cesium, Chlorine, Magnesium, and Oxygen. Which atom in each bond will be more negative?
The electronegativity of Sulfur is 2,5 (see Appendix 7). The more electronegative end of atom in each bond will be the atom with the larger electronegativity.
| Bond from Sulfur to | Electronegativity difference | Bond type | More electronegative atom |
| Hydrogen | 2,5 - 2,1 = 0,4 | Polar covalent | Sulfur |
| Cesium | 2,5 - 0,7 = 1,8 | Ionic | Sulfur |
| Chlorine | 3,0 - 2,5 = 0,5 | Polar covalent | Chlorine |
| Magnesium | 2,5 - 1,2 = 1,3 | Polar covalent | Sulfur |
| Oxygen | 3,5 - 2,5 = 1,0 | Polar covalent | Oxygen |
Date: 2015-01-12; view: 4646
| <== previous page | | | next page ==> |
| The Periodic Table | | | Figure 10. Formation of Hydrogen Bonds between water molecules |