
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Experiment 8
ELEMENTS OF VA AND VIA GROUPS
(P-ELEMENTS)
Nitrogen and phosphor are elements of VA group of the periodic table. They have 5 electrons in the outer shell (valence electrons). They may lose electrons and form positively charged ions (oxidation numbers from +1 to +5) or gain electrons and form negatively charged ions (oxidation number -3).
Hydrogen compounds of nitrogen and phosphor are ammonia NH3 and phosphine (PH3). The presence of a lone electron pair of nitrogen and phosphor leads to the possibility of formation of a coordinate bond with a proton. In aqueous solutions ammonia interacts with water molecules to form ammonium hydroxide which possesses weak basic properties:
NH3 + H2O  NH4OH
NH4OH  NH4+ + OH
NH4+ + OH 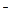
Ammonium ion has almost same properties as metallic ions, for example it can form salts. Ammonium salts decompose while heating:
NH4Cl  NH3 + HCl
NH3 + HCl
In oxides nitrogen has various oxidation states from +1 to +5. Nitric acid HNO3 is one of the strongest acids with a high oxidizing strength. Depending on the nature of a reducing agent and the concentration of the acid, the NO3 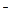 group can gain from 1 to 8 electrons and transfer into NO2, NO, N2O, N2 or NH4+.
group can gain from 1 to 8 electrons and transfer into NO2, NO, N2O, N2 or NH4+.
Salts of nitric acid (nitrates) are water soluble.
Nitrous acid HNO2 is a weak acid with redox duality. It exists only in diluted solutions and decomposes at high concentrations:
3HNO2 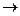 HNO3 + 2NO
HNO3 + 2NO 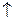 + H2O
+ H2O
In contrast with nitric acid, phosphoric acid has no oxidizing properties. Phosphates form soluble complexes with a lot of metal ions.
Sulfur is situated in the VIA group of the periodic table and has 6 valence electrons. Its oxidation numbers are +4 , +6 and -2. Sulfuric acid H2SO4 (conc.) is a strong oxidizing agent and hydrogen sulfide and its salts (sulfides) are good reducing agents. Sulfurous acid H2SO3 and its salts (sulfites) have redox duality.
The strength of acids containing sulfur increases with the increase in oxidation number of sulfur.
Biological importance of sulfur
Sulfur is a composite of most important aminoacids. Metal sulfates are used in medicine: CaSO4 as a stuff for plasters, BaSO4 in rontgenoscopy of stomach, MgSO4´10H2O as purgative. Some antibiotics have sulfur compounds as composites.
EXPERIMENTAL PART
1. Formation and properties of ammonia
Take equal quantities of dry ammonium chloride and calcium hydroxide and mix them in a porcelain mortar. Transfer the mixture in a dry test tube, cover with a cork with a tube and heat. Collect ammonia in a dry test tube holding it with its bottom upwards. Study properties of ammonia: a) Immerse the test tube filled with ammonia into a cup with water with 2 drops of phenolphtalein (hold it with the bottom upwards). Write down your observations.
b) Take some anhydrous copper sulphate in a dry test tube and pass ammonia from the tube. Observe the formation of a complex compound of tetraamminecopper (II) sulphate.
c) Hold a stirring rod moistened with hydrochloric acid in front of the test tube filled with ammonia. Observe the formation of a “smoke” of ammonium chloride.
2. Oxidizing properties of nitric acid
Heat carefully a test tube with 2-3 ml of concentrated nitric acid and add a smouldered piece of coal. Observe evaluation of a brown gas of NO2. Write down and balance the reaction.
3. Redox duality of nitrites
a) Take 1 ml of sodium nitrite in a test tube, add 1 ml of potassium iodide and 1 ml of 2 N sulfuric acid. Observe evaluation of iodine.
b) Take 1 ml of sodium nitrite into a test tube, add 1 ml of 2 N sulfuric acid and 5 drops of potassium permanganate.
Write down and balance the reactions (a) and (b).
4. Reducing properties of sulfides
Take 1 ml of an aqueous solution of iodine and add some drops of potassium sulfide solution. Write down and balance the reaction.
5. Formation of sulfides
Take 5 test tubes filled with Zn, Mn(II), Cd, Sn(II) and Pb(II) salts respectively and add potassium sulfide into each test tube. Observe colors of the formed sulfides.
6. Reducing properties of sulfites
Take 1 ml of potassium dichromate into a test tube, add sulfuric acid and sodium sulfite solution. Write down and balance the reaction.
7. Oxidizing properties of sulfuric acid
Take 2 ml of concentrated sulfuric acid in the test tube (be careful!) and add some pieces of copper. Heat the test tube if necessary. Observe the evaluation of sulfur dioxide. Write down and balance the reaction.
QUESTIONS AND PROBLEMS
1. Describe the electronic structure of ammonia and NH4+ ion.
2. Using the molecular orbital method draw molecular diagrams of N2 and O2 molecules. Compare stabilities of these molecules.
3. Calculate pH of 0.01 M solution of NH4OH.
4. Calculate the solubility of Ag2SO4: (a) in pure water; (b) in presence of 0.1 M solution of sulfuric acid.
5. Compare solubilities of MnS and CuS in diluted hydrochloric acid.
Date: 2015-01-12; view: 1911
| <== previous page | | | next page ==> |
| Experiment 7 | | | CHEMISTRY |