
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Experiment 7
ELEMENTS OF IIIA AND IVA GROUPS
(P - ELEMENTS)
Boron and aluminum are elements of the IIIA group of the periodic table. Atomic radius of boron is 0.91Å and the one of aluminum is 1.43Å. This great difference affects chemical properties of these elements. Ionization potential of boron is greater than that of aluminum. Polarity of B-O chemical bond is small, so in solutions boron exists as BO2 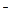 and BO33
and BO33 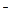 ions (acidic properties). Al-O chemical bonds have a more polar character, so in solutions aluminum exists both as Al3+ and AlO2
ions (acidic properties). Al-O chemical bonds have a more polar character, so in solutions aluminum exists both as Al3+ and AlO2 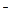 ions (amphoteric properties).
ions (amphoteric properties).
Salts of boric acid H3BO3 are metaborates (Ba(BO2)2) and tetraborates (Na2B4O7 - borax).
Biological properties of boron and aluminum
Physiological activity of boron is rather high. Together with Mn, Cu, Zn and Mo it is among five most important microelements. It concentrates in bones, teeth, muscles, marrow, liver and thyroid gland, can be found in adipose tissues of some animals, in milk and yolk of eggs.
Boron inhibits the action of amilaze and proteinaze, vitamins B2 and B12, reinforces the action of insuline. Boric acid and borax are used in medicine as anticeptics.
Some compounds of aluminum are also used in medicine: KAl(SO4)2 as astringent; AlOH(CH3COO)2 for desinfection; Al2(SO4)3 as coagulant.
Carbon and silicon are elements of IVA group of the periodic table of elements. Their highest oxidation number is +4.
At a room temperature carbon and silicon are inert elements, their activities increase with heating. At high temperatures they react with the majority of non-metals and metals.
Concentrated nitric and sulfuric acids oxidize carbon into CO2, silicon can be oxidized by mixture of HNO3 and HF. Silicon can also be dissolved in alkalis:
Si + 2NaOH 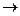 Na2SiO3 + 2H2
Na2SiO3 + 2H2
Carbon (II) oxide CO is a non-salt forming oxide. Carbon dioxide CO2 has acidic properties and reversibly dissolves in water to form a weak carbonic acid:
CO2 + H2O  H2CO3
H2CO3  H+ + HCO3
H+ + HCO3 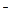
Salts of carbonic acid are carbonates (Na2CO3) and hydrocarbonates (NaHCO3).
In aqueous solutions carbonates and hydrocarbonates undergo hydrolysis:
Na2CO3 + H2O  NaHCO3 + NaOH
NaHCO3 + NaOH
Silicic acid is even weaker than carbonic acid, the reactions of hydrolysis of its salts lead to the formation of polyanions:
2Na2SiO3 + H2O  Na2Si2O5 + 2NaOH
Na2Si2O5 + 2NaOH
EXPERIMENTAL PART
1. Properties of boric acid and its salts
Take 0.5 g of boric acid or borax in a porcelain dish and add 2 ml of concentrated sulfuric acid (be careful!). Stir the mixture with a glass rod and add 3 ml of ethanol. The reaction of formation of boric ester of ethanol takes place:
H3BO3 + 3C2H5OH 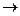 B(OC2H5)3 + 3H2O
B(OC2H5)3 + 3H2O
The ester burns with a green flame. This reaction can be used as an analytical reaction of boric acid and its salts.
2. Hydrolysis of Na2B4O7
Dissolve some crystals of Na2B4O7 in water and add 1 drop of phenolphtalein. Write the reaction of hydrolysis of Na2B4O7 in molecular and net ionic forms.
3. Reaction of aluminum with acids and bases
a) Take 3 pieces of aluminum in separate test tubes and add HCl, HNO3(dil.) and HNO3(conc.) respectively. Write down and balance the corresponding reactions (if possible).
b) Take a piece of aluminum in a test tube, add NaOH solution and heat carefully. Observe the evaluation of hydrogen. Write down and balance the reaction of interaction of Al with NaOH.
5. Formation and properties of aluminum hydroxide
Take some solution of any aluminum salt in a test tube and add NaOH dropwise until the precipitate of aluminum hydroxide is formed. Divide the precipitate into two parts and add NaOH and HCl respectively. Write down the molecular and net ionic reactions. Make the conclusion about the properties of aluminum hydroxide.
6. Hydrolysis of aluminum salts
a) Dissolve some crystals of AlCl3 in water and measure pH with the help of a universal indicator paper. Write the reaction of hydrolysis of AlCl3 in molecular and net ionic forms.
b) Mix the solutions of AlCl3 and Na2CO3 in one test tube. Observe the formation of a precipitate of Al(OH)3. Explain the phenomenon of mutual hydrolysis of these salts.
7. Formation of carbon dioxide
Take some pieces of marble (CaCO3) in a Kipp apparatus and add HCl. Observe the formation of carbon dioxide. Pass it through the test tube filled with distilled water. Measure pH of the saturated solution of carbon dioxide and make your conclusions about properties of aqueous solutions of this oxide.
8. Formation of calcium carbonate and hydrocarbonate
Pass carbon dioxide from Kipp apparatus through the saturated calcium hydroxide solution. Observe the formation of calcium carbonate precipitate and its dissolution in excess of CO2 with the formation of calcium hydrocarbonate.
9. Hydrolysis of carbonates
Dissolve some crystals of Na2CO3 and (NH4)2CO3 in water and measure pH of the solutions with the help of a universal indicator paper. Write the reaction of hydrolysis of these salts in molecular and net ionic forms.
10. Formation of silicic acid
Take 3 ml of a solution of sodium silicate in a test tube and add 1 ml of diluted HCl. Stir the solution and observe the formation of gel of silicic acid.
QUESTIONS AND PROBLEMS
1. Which are oxidation states of elements of IIIA and IVA groups of the periodic table? Write their electronic structures and mark valence electrons.
2. Which are chemical properties of oxides of boron, aluminum, carbon and silicon. Using table data, compare strengths of corresponding acids.
3. Prove amphoteric properties of aluminum hydroxide.
4. Calculate pH of 0.1 M solution of NaHCO3.
5. Calculate the equilibrium constant of a reaction and explain if the precipitate of calcium carbonate can be dissolved in acetic acid.
Date: 2015-01-12; view: 1525
| <== previous page | | | next page ==> |
| COMPLEX (COORDINATE) COMPOUNDS | | | Experiment 8 |