
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Involving Two or More Substrates
Thus far, we have considered only the simple case of enzymes that act upon a single substrate, S. This situation is not common. Usually, enzymes catalyze reactions in which two (or even more) substrates take part.
Consider the case of an enzyme catalyzing a reaction involving two substrates, A and B, and yielding the products P and Q:
 (14.45)
(14.45)
Such a reaction is termed a bisubstrate reaction. In general, bisubstrate reactions proceed by one of two possible routes:
1. Both A and B are bound to the enzyme and then reaction occurs to give P1Q:
 (14.46)
(14.46)
Reactions of this type are defined as sequential or single-displacement reactions. They can be either of two distinct classes:
a. random, where either A or B may bind to the enzyme first, followed by the other substrate, or
b. ordered, where A, designated the leading substrate, must bind to E first before B can be bound.
Both classes of single-displacement reactions are characterized by lines that intersect to the left of the 1/v axis in Lineweaver-Burk double-reciprocal plots (Figure 14.18).

Figure 14.18 •Single-displacement bisubstrate mechanism. Double-reciprocal plots of the rates observed with different fixed concentrations of one substrate (B here) are graphed versus a series of concentrations of A. Note that, in these Lineweaver-Burk plots for single-displacement bisubstrate mechanisms, the lines intersect to the left of the 1/v axis.
2. The other general possibility is that one substrate, A, binds to the enzyme and reacts with it to yield a chemically modified form of the enzyme (E') plus the product, P. The second substrate, B, then reacts with E', regenerating E and forming the other product, Q.
 (14.47)
(14.47)
Reactions that fit this model are called ping-pong or double-displacement reactions. Two distinctive features of this mechanism are the obligatory formation of a modified enzyme intermediate, E', and the pattern of parallel lines obtained in double-reciprocal plots (Figure 14.19).
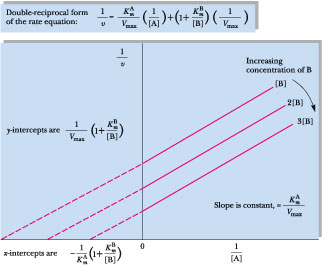 Figure 14.19 • Double-displacement (ping-pong) bisubstrate mechanisms are characterized by Lineweaver-Burk plots of parallel lines when double-reciprocal plots of the rates observed with different fixed concentrations of the second substrate, B, are graphed versus a series of concentrations of A.
Figure 14.19 • Double-displacement (ping-pong) bisubstrate mechanisms are characterized by Lineweaver-Burk plots of parallel lines when double-reciprocal plots of the rates observed with different fixed concentrations of the second substrate, B, are graphed versus a series of concentrations of A.
Date: 2016-01-03; view: 1031
| <== previous page | | | next page ==> |
| Competitive Inhibition | | | Diagnosis of Bisubstrate Mechanisms |