
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Diagnosis of Bisubstrate Mechanisms
Kineticists rely on a number of diagnostic tests for the assignment of a reaction mechanism to a specific enzyme. One is the graphic analysis of the kinetic patterns observed. It is usually easy to distinguish between single- and double-displacement reactions in this manner, and examining competitive effects between substrates aids in assigning reactions to random versus ordered patterns of S-binding. A second diagnostic test is to determine whether the enzyme catalyzes an exchange reaction. Consider as an example the two enzymes sucrose phosphorylase and maltose phosphorylase. Both catalyze the phosphorolysis of a disaccharide and both yield glucose-1-phosphate and a free hexose:
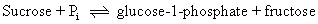
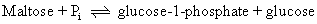
Interestingly, in the absence of sucrose and fructose, sucrose phosphorylase will catalyze the exchange of inorganic phosphate, Pi, into glucose-1-phosphate. This reaction can be followed by using 32Pi as a radioactive tracer and observing the appearance of 32P into glucose-1-phosphate:
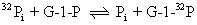
Maltose phosphorylase cannot carry out a similar reaction. The 32P exchange reaction of sucrose phosphorylase is accounted for by a double-displacement mechanism where E' = E-glucose:
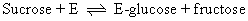
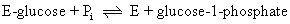
Thus, in the presence of just 32Pi and glucose-1-phosphate, sucrose phosphory-lase still catalyzes the second reaction and radioactive Pi is incorporated into glucose-1-phosphate over time.
Maltose phosphorylase proceeds via a single-displacement reaction that necessarily requires the formation of a ternary maltose: E : Pi (or glucose: E: glucose-1-phosphate) complex for any reaction to occur. Exchange reactions are a characteristic of enzymes that obey double-displacement mechanisms at some point in their catalysis.
Date: 2016-01-03; view: 919
| <== previous page | | | next page ==> |
| Involving Two or More Substrates | | | Multisubstrate Reactions |