
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Synthetic procedures
Once the chemist has decided which route to follow, he has to anticipate where problems might arise. Each step must be examined to make sure it will proceed as planned. Also, the chemist must consider whether a particular step might affect the wrong part of the molecule. If this is likely, he may be able to shield the molecule with what is termed a "protecting group." This temporarily denies reagents access to groups in the molecule that are prone to attack. It can be removed to restore the original grouping at a later stage.
The chemist also has to consider which steps may be impossible for practical reasons. For example, he may be unable to achieve the pressures needed for one stage. Or the reaction may be too explosive to risk in the laboratory. The more complex the product required, the more problems face the organic chemist. As the number of steps in a reaction increases, so too do the number of options. Also, the problems of unwanted side reactions multiply as the size of the molecule—and hence the number of sites prone to attack—increases. It is, therefore, not surprising that many satisfactory synthetic routes to complex products take years to perfect.
New developments
Advances in computer technology are making synthesis much easier. Much information can be stored and processed by computers. This includes details of synthetic methods, alternative methods of changing chemical groupings, and data on how different chemical agents react in different chemical reactions.
Computers are also valuable in scaling-up and processing operations. Traditionally, scale-up follows a familiar pattern. Having established the need to make a particular product, one or two synthetic routes are investigated. The most favorable route is then developed into a process that can be carried out in a production (scaled-up) environment.
Computers can also be used to regulate and monitor reaction conditions. Remote operation provided by a computer can also permit safe handling of toxic raw materials, intermediates, and products. Hazardous reactions can also be safely supervised from a distance.
Protecting groupsplay an essential part in many organic synthesis reactions, as demonstrated in the example. It is impossible to convert compound 1 into compound 4 directly by simply adding a hydroxyl group (OH) at A. This is because the hydroxyls prefer to add themselves to the double bond at positions B and C. These vulnerable sites are therefore protected by adding bromine (Br), to give compound 2. The hydroxyl group can then be added at the desired site, forming compound 3. Finally, the bromine atoms are removed to restore the double bond and give the required compound (4).
| jf |
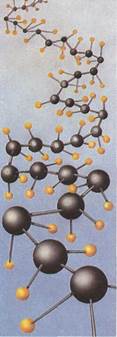
Polymersare giant molecules formed from many small units. One of the simplest is polyethylene. This polymer consists of long chains of carbon atoms (black), each carrying two hydrogen atoms (yellow).
Polymers
Polymeric materials are composed of very large molecules (macromolecules) formed by linking together many smaller, more simple units called monomers. The process of linking together the monomers and making polymers is called polymerization.
There can be as few as five or as many as several thousand monomer units in a polymer. Typical examples of polymers are plastics: polyethylene film, a transparent material used in packaging; polyurethane foam made into cushions and mattresses; and nylon and polyester fibers used in textiles. Synthetic (artificial) resins for paints and adhesives are also polymers. Rubber is a natural polymer isolated from trees native to South America, but now grown mostly in Asia. However, more than half the rubber used today is synthetic. Other natural polymers include various protein substances and plant carbohydrates, such as starch and cellulose.
Scientists have a broad range of monomers at their disposal. They can modify the monomers and the polymerization conditions. This allows them to make synthetic polymers that suit particular needs and exhibit special properties. These can also vary depending on the number of monomer units in each macro-molecule and the way they link together. Branched-chain polymers are normally stronger than straight-chain polymers, because of the extra linkages between chains. Polymers can also respond differently to the action of heat. Thermoplastic polymers can be molded again if remelted, whereas thermosetting polymers harden permanently once molded.
If a single substance is used for the monomers, the resulting polymer is called a homo-polymer. Copolymers are formed by linking
together different kinds of monomers. Reactions to combine monomeric units can also vary. In condensation polymerization, a small molecule such as water is ejected by monomers as they come together. Addition polymers are formed by direct linkage of monomer units.
Polyethylene
Perhaps the most familiar of all polymers, polyethylene exists in various forms according to the way it is manufactured. Low-density polyethylene (LDPE) was the first type invented. It is produced by heating ethene to about ZOO-GOO0 F. (about 100-300° C) under a pressure of 1,000-2,000 atmospheres. During polymerization, some chains branch slightly so the molecules cannot pack closely—hence its low density. It is a soft substance that can be used as packaging material and for plastic bags, squeeze bottles, and so forth. By contrast, high-density polyethylene (HDPE) is composed of close-fitting chains. This makes it ideal for blow-molded and injection-molded products. It is made by subjecting ethene in solution to a pressure of 5-30 atmospheres in the presence of a catalyst that contains, for example, aluminum and titanium. Low-molecular weight polyethylene (LMVVPE) has good electrical resistance plus excellent resistance to chemicals and abrasion. It is used in paper and container coatings, liquid polishes, and textile finishing agents. Its molecular weight ranges between 1,000 and 10,000. The other varieties of polyethylene generally have a molecular weight between 10,000 and 400,000. Certain varieties may reach as much as 1,000,000.
Polypropylene
Polypropylene is a thermoplastic polymer. It is soft and capable of being molded when heated. Its molecular weight is usually higher
 | |||||
 | |||||
 | |||||

|

|
 One of the main methods
One of the main methods
of polymerization involves a chain reaction triggered by the presence of a free radical. This is a highly reactive molecule bearing an unpaired electron (shown by the blue dot). Polymerization usually starts with an alkyl peroxide, which decomposes into free radicals on heating (top). These combine with a monomer to form more free radicals. In turn these compounds react with more monomer molecules to build up a long chain (middle). Finally, two long-chain components combine to form a molecule of polymer and so terminate the reaction (bottom).
Initiation (start of reaction)
Alkyl peroxide
Monomer
Propagation (chain reaction)
r
Free radicals
Free radical
Heat splits alkyl peroxide molecule into two free radicals
Free radical combines with monomer to form another, longer free radical

Long-chain free radical
Monomer
Longer-chain free radical


Termination (end of reaction)
Two longer-chain free radicals
Organic chemistry: Polymers 101
 | |||
 |

|
than that of polyethylene. There are three forms, which differ according to the arrangement in which the monomer units are put together. The arrangement has a marked effect on the properties of the plastic and accounts for its wide range of uses, from carpets to containers. By employing special catalysts, polymer scientists are able to link the monomeric propene (propylene) units in regular sequences, which impart more mechanical strength to the polymer. The second form has a crystalline structure. It is used in a variety of blow-molded items and for packaging film. Atactic (disordered) polypropylene is more pliable and semitacky. It can be used with bitumen on roofs and roads and in backings for carpet tiles.
Polystyrene
This material is renowned for its use as a thermal and electrical insulation. It protects against the loss of heat and prevents the transfer of electricity. Polystyrene is a thermoplastic resin made from phenylethene (styrene), a type of liquid hydrocarbon. Perhaps most familiar as rigid foam or as expandable beads, it is used in packaging, ice buckets, water coolers, furniture construction, thermal insulation, ceiling tiles, and a variety of other items. The most common use for solid polystyrene is in making ballpoint pens. A special property of branched—as opposed to linear—polystyrene is its insolubility. Instead of dissolving in a solvent, it swells up. The degree of swelling depends on the degree of cross-linking. It thus forms a resin.
Date: 2015-12-11; view: 3374
| <== previous page | | | next page ==> |
| Strategies of synthesis | | | Halogenated polymers |