
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Strategies of synthesis
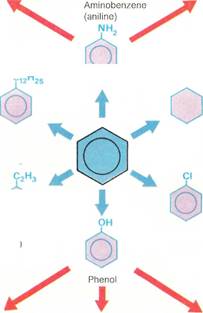
Once a chemist has decided upon the need to synthesize a product, he has to work out a satisfactory synthetic route by which to make it. The usual strategy is to work backward from the required product. For instance, if a complex product Z is required, it may be possible to make it by modifying substance Y, from which Z is then made. Y, in turn, may be available from X, and so on. This continues until the chemist reaches starting materials that are readily available and cheap. Because each compound on the way to the final product can usually be synthesized by a variety of methods, the scope for developing an array of reaction pathways is almost limitless. Essentially, the chemist usually has to change one part of the molecule at a time, while leaving the rest of it undisturbed.
So, in deciding upon which synthetic route to choose, a number of factors have to be weighed against one another. The route that uses the cheapest and most widely available starting materials might seem to be preferable, especially in industrial synthesis. But this is not always the most convenient route from a chemist's point of view. It may involve dangerous reactions. Perhaps some of the steps give only small amounts of the product needed for the next stage of the synthesis (the so-called intermediates). This also means wastage of starting materials. However, in some industrial syn-
 Synthetic dyes j e.g. diazo dyes
Synthetic dyes j e.g. diazo dyes
 |
|

|

|

|
| Dodecylbenzene |
| Cyclohexane |
| Polymers e.g. .^ f / ^V| polystyrene ^ Phenylethene (styrene |
| Chlorobenzene |

Phenolic resins e.g. Bakelite
-:-'■..... '■.......... ;'::■■■ :■■■> :"::s::s........ !■;■;:;■.;:;:;,;:.:-:.:,:
Pesticides e.g. DDT
Pesticides and herbicides e.g. 2,4-D
Organic chemistry: Organic synthesis 99

|
Spools of dyed fibersare
being made into a carpet. Most modern dyes used to color fibers and textiles —and many of the fibers and textiles themselves—are synthetic. They are made from starting materials such as benzene and other aromatic hydrocarbons.

|
theses, it may be possible to recycle starting materials and intermediates. Thus, the effective yield is increased.
The amount produced in each stage is indicated by the yield. The yield is the percentage of starting material in that particular step that is converted into the required intermediate. Other problems might arise in purification. Starting materials and unwanted by-products may contaminate the product. Therefore, if the intermediate yields are low, the synthesis as a whole may be expensive.
A further drawback may be that some of the steps proceed very slowly. The chemist may try to speed them up by raising the temperature of the reaction or by using a catalyst, a substance that speeds up chemical reactions. But care is needed to avoid destroying the product or synthesizing other, unwanted substances.
Date: 2015-12-11; view: 3214
| <== previous page | | | next page ==> |
| Artificial sweeteners and flavorings | | | Synthetic procedures |