
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Artificial sweeteners and flavorings
By far, the best-known artificial sweetener is saccharin. Some experiments with animals have led to the suspicion that it may cause
cancer. Other synthetic sweeteners, sodium and calcium cyclamate, were banned in 1970. They were found to cause bladder cancer in rats. Scientists are now developing new sweeteners. One type is nearly one thousand times sweeter than ordinary sugar (sucrose). Saccharin is only 300 times sweeter. Other artificial sweeteners include Acesulfame-K, which does not accumulate in the body, and aspartame.
|
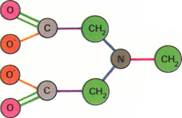
| Ethylenediamine tetraacetate (EDTA) |
| (ethenediamine tetra-ethanoate) |
| EDTA-cobalt complex |
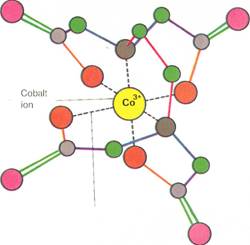
|
| Coordinate bond |
About 800 synthetic food flavorings are now also in use. A common flavor enhancer is monosodium glutamate. It is usually isolated from natural sources—such as flour or soybean—fermented, and then purified.
(Rh—©
(ch)--- 0
©
Chelating agents,such as EDTA illustrated above, can form coordinate bonds with metals. This produces coordination compounds, also called complexes. The diagram left shows how a molecule of EDTA wraps around and forms coordinate bonds (the dotted lines) with a cobalt ion. Chelating agents are useful in removing metallic poisons, such as lead, from the body.
 Color in organic chemistry
Color in organic chemistry
 |
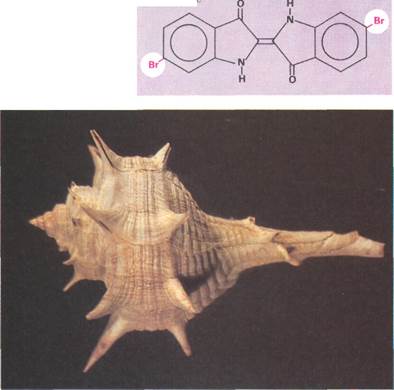 Indigo and Tyrian purple
Indigo and Tyrian purple
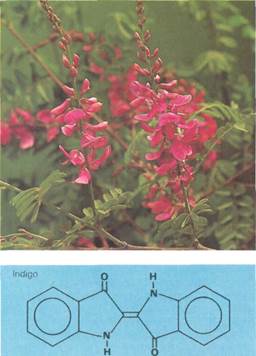
have both been used as dyes since ancient times. Indigo was originally obtained from various species of the indigo plant (Indigo-fera sp.l, principally from /;?-digofera tinctoha (top right). This plant is native to parts of eastern Asia and Central America. In 1897, however, the dye was synthesized. Today, almost all indigo is obtained synthetically. The structure of indigo is very similar to that of Tyrian pur pie. The only difference is that Tyrian purple contains two bromine (Br) atoms. This can be seen by comparing the structures in the diagram (middle right). The presence of these bromines has the effect of displacing the color of the compound toward the red. This results in the purple color of Tyrian purple as opposed to the dark blue of indigo. Like indigo, Tyrian purple was originally obtained from a natural source, the aquatic mollusk Murex brandaris (the shell of which is shown below). Today, this mollusk has been superseded by synthetic dyes.
| Tyrian purpj |
Many organic compounds are brightly colored. Some, such as the green plant pigment chlorophyll, occur naturally. Others are synthesized by chemists for use as dyes and colorants. All colored organic compounds share one feature. This is the existence in their structures of sequences of atoms linked by double bonds, either in chains or rings. It is these se-
quences (called chromophores) that are responsible for absorbing specific wavelengths of light. Light travels in waves. Wavelength refers to the distance between wave peaks. Each color is linked to a specific wavelength. The wavelengths that are reflected make the compound appear to have the color associated with those wavelengths. By altering the number and sequence of doubly-bonded atoms, chemists can deliberately vary the colors of compounds.
Natural pigments
Many of the naturally occurring organic pigments show similarities in their molecular structures. They are all aromatic compounds with oxygen atoms in their structures. Natural pigments account for most of the yellow, red, and blue colors in flowers and fruits. They may be divided into various subgroups. The cat-echins (yellow, powdery acid compounds) are used as dyestuffs and in special inks. They turn brown in color on exposure to air and light.
Another group of natural pigments are the Ieucoanthocyanidins. Indigo, the dye used in blue jeans, is probably the best known. It can be prepared easily from a compound called indican, which is found in the indigo plant. This accounts for the wide use of indigo as a dyestuff since ancient times.
A third group contains flavonols, flavones, and anthocyanins. Flavonols and flavones account for the ivory and yellow colors of many plants. Anthocyanins are responsible for most of the red and blue colors. The particular color imparted by an anthocyanin compound depends on the acidity or alkalinity in the plant. For example, cyanin is the pigment in red roses and blue cornflowers. It is red in dilute acid and blue in dilute alkali.
Another group includes the naphthoquinones and anthraquinones. Probably the most familiar example of the former is lawsone, the substance in the leaves of the henna shrub. This substance gives the orange-red color to henna dye. Carminic acid is a typical anthra-quinone. It is the principal pigment in cochineal. This is the scarlet dye obtained from the dried, pulverized bodies of the insect Coccus cacti. Carminic acid is widely used in food and cosmetics.
Other common natural pigments include chlorophyll, carotene, and rhodopsin. Chlorophyll plays a key role in photosynthesis, the process by which plants harness the energy of the sun to manufacture nutrients. Carotenoids are responsible for the orange, yellow, and red pigments in many flowers and animals. A well-known example is beta-carotene, which imparts the orange color to carrots. Rhodopsin, otherwise known as visual purple, occurs in the retina of the eye and is involved in triggering the visual process.
Azo dyestuffs
Azo dyes are distinguished by the presence in their structures of at least one nitrogen-nitrogen double bond. They are produced artifi-
Organic chemistry: Color in organic chemistry 97

|
daily (synthesized) in the laboratory. Perhaps the best-known azo dye is Congo red, a brown-red compound. Another is butter yellow, a vibrant orange substance. It was once used as a food additive until suspected of causing cancer.
Many of the most widely used azo dyes contain sulfur (in the form of sulfonate groups) in their structures. Sulfonate groups make the dyes soluble in water. They also serve to bind the dyes tightly to the large complex molecules of textiles. Because some azo dyes change color when reacted with an acid or base, they are used by chemists to help identify substances present in a solution. The color change is abrupt and is caused by the conversion from an acid into a base or vice versa.
Metal-organic dyes
A number of important metal-organic dyes are based on the compound phthalocyanine, a blue-green compound that itself is not bound to a metal. Binding phthalocyanine to copper gives a striking blue dye. By replacing hydrogen atoms in copper phthalocyanine with chlorine atoms, it is possible to form a bright green compound. This is called chlorinated copper phthalocyanine. Both these blue and green compounds are used in paints, printing inks, resins, colored chalks, and pencils. Other uses for these dyes include decorative enamels and paints for motor vehicles.
Food colorants
To be accepted for use as a food colorant, a compound must satisfy strict regulations relating to its toxicity (poisonous qualities). Indeed, many compounds formerly used as food colorants have now been banned because they fail to satisfy current regulations. An example is amaranth ("Red No. 2"). At one time, amaranth was the most widely used food colorant. It is now thought to cause cancer and has been banned from use in food by the United States Food and Drug Administration.
The brilliant orange-yellow robesof Buddhist monks are traditionally dyed using the dried stigmas of the saffron plant, a species of crocus (Crocus sativus). The active constituent of this plant is a volatile oil called picrocrocin. In addition to being employed as a coloring agent, saffron is also used for flavoring. It imparts a bitter, aromatic taste to food.
Inksare available in a wide range of colors—as exemplified by the felt-tip pens above—and many of the dyes used in them are synthetic organic compounds, principally azo dyes and metal-organic dyes. The former are derivatives of azo-benzene. They are usually red, yellow, or brown. The main metal-organic dyes are synthesized from the blue-green compound phthalocyanine.

|
 The table lamp(belowleft) dates from the late 1920s. It is made of Bakelite, the first successful plastic to be produced (in the early 1900's). It is formed by the condensation reaction between phenol (usually synthesized from benzene) and metha-nal (formaldehyde). The resultant phenolic resin is then melted and allowed to set. This process makes the material extremely hard and unmeltable on further heating. Phenolic resins such as Bakelite are only one of the many types of chemicals that can be synthesized from benzene. Some of its other important synthetic derivatives are shown in the diagram (below right).
The table lamp(belowleft) dates from the late 1920s. It is made of Bakelite, the first successful plastic to be produced (in the early 1900's). It is formed by the condensation reaction between phenol (usually synthesized from benzene) and metha-nal (formaldehyde). The resultant phenolic resin is then melted and allowed to set. This process makes the material extremely hard and unmeltable on further heating. Phenolic resins such as Bakelite are only one of the many types of chemicals that can be synthesized from benzene. Some of its other important synthetic derivatives are shown in the diagram (below right).
Organic synthesis
Organic chemists can construct complex organic compounds from simpler ones using a variety of reactions. These reactions are the techniques of organic synthesis, the artificial production of organic compounds. To make just one compound, a chemist may have to perform a long series of reactions using many different reagents. Reagents are chemical substances used to start or change chemical reactions. There may also be several ways of making any given compound.
In choosing which synthetic (artificial) route or pathway to adopt, the chemist has to compromise between various factors. These include: which reaction procedures are simplest and most convenient; which reagents are cheapest or most widely available; and which reactions give the best yields of compounds to be used in the next step of the synthesis.
The reasons for doing a synthesis are threefold. One is that particular organic products may be in demand commercially. Examples are drugs, which are often synthesized by carefully constructed and elegant synthetic routes. Another reason is that a chemist may wish to establish the structure and identity of a natural product. A great many of these are isolated from plants and animals. Because the natural products may possess valuable properties (perhaps medicinal or industrial), it is important for scientists to know their structures. They do this by synthesizing compounds (often with much trial and error) that correspond exactly with the natural product, both in chemical reactions and physical properties. If the compound is shown without a doubt to be
identical with the natural product, then this confirms what its structure is. The third reason is to make completely new compounds. This is done to test theories of reactivity in chemistry. New compounds may also turn out to have important properties.
Date: 2015-12-11; view: 1819
| <== previous page | | | next page ==> |
| Synthetic pesticides | | | Strategies of synthesis |