
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
FACTORS INFLUENCING BACTERIAL GROWTH 305
|
| TABLE 10-4 |
salt concentrations. The degree of sensitivity to salt varies for different bacterial species. Many bacteria will not grow at a salt concentration of 3%. Some strains of Staphylococcus, however, are salt tolerantand grow at concentrations greater than 10% NaCl. This physiological adaptation in Staphylococcus is important because some members of this genus grow on skin surfaces where salt concentrations can be relatively high.
Acidity and pH
The pH of a solution describes the hydrogen ion concentration([H+]). When bacteria are cultured in the laboratory, they produce acids that can interfere with their own growth. To neutralize the acids and maintain the proper pH, chemicals called buffersare included in the growth medium. Peptones and amino acids in some media act as buffers. Many media also contain phosphate salts, which exhibit their buffering effect in the pH growth range of most bacteria. They are also nontoxic and even provide phosphorus, an essential nutrient element.
Buffers neutralize acids and maintain the proper pH.
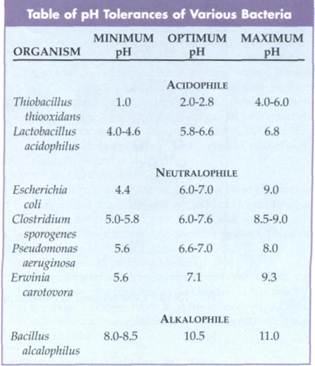
Microorganisms vary in their pH tolerance ranges (FIG. 10-17). The pH is equal to log [H+] or 1/log [H+]. A neutral solution has a pH of 7.0; acidic solutions have pH values less than 7; and alkaline or basic solutions have pH values greater than 7. Most micro-organisms grow best at near neutral pH. These microorganisms are called neutralophiles or neu-trophiles. Fungi generally exhibit a wider pH range, growing well over a pH range of 5 to 9, compared to most bacteria, which grow well over a pH range of 6 to 9 (Table 10-4).
Although most bacteria are unable to grow at low pH, there are some exceptional cases. Some bacteria tolerate pH values as low as 0.8. There are even some bacteria, called acidophiles,that are restricted to growth at low pH values. Some members of the genus Thiobacillus are acidophilic and grow only at pH values near 2; they can grow in sulfuric acid.
Alkalophilesare microorganisms that prefer to grow at high pH values. These microorganisms are found in alkaline environments that are high in sodium such as salt lakes and soils rich in sodium carbonate. The alkalophile Bacillus alkalophilus grows best at pH 10.5.
Pressure
The solute concentration of a solution affects the osmotic pressure that is exerted across the plasma membrane. The cell walls of bacteria make them relatively resistant to changes in osmotic pressure, but
extreme osmotic pressures can result in the death of bacterial cells. In hypertonicsolutions, bacterial cells may shrink and become desiccated; in hypotonicsolutions, the cell may burst (FIG. 10-18). Bacteria that can grow in solutions with high solute concentrations are called osmotolerant.These bacteria can withstand high osmotic pressures. Some fungi, such as Xeromyces, are actually osmophilic.
Hydrostatic pressure is another type of pressure that can influence bacterial growth rates. Hydrostatic pressurerefers to the pressure exerted by a water column as a result of the weight of the water column. Each 10 meters of water depth is equivalent to approximately 1 atmosphere pressure. Most bacteria are relatively tolerant to the hydrostatic pressures in most natural systems but cannot tolerate the extremely high hydrostatic pressures that characterize deep ocean regions. High hydrostatic pressures of greater than 200 atmospheres generally inactivate enzymes and disrupt membrane transport processes. However, some bacteria — referred to as barotolerant—can grow at high hydrostatic pressures, and there even appear to be some bacteria — referred to as barophiles—that grow best at high hydrostatic pressures.
Light Radiation
Photosynthetic microorganisms require light in the visible spectrum to carry out photosynthesis. The rate of photosynthesis is a function of light intensity. At some light intensities, rates of photosynthesis
Date: 2015-02-28; view: 1539
| <== previous page | | | next page ==> |
| Growing Cultures of Aerobic and Anaerobic Bacteria | | | CHAPTER 10 BACTERIAL REPRODUCTION AND GROWTH OF MICROORGANISMS |