
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Growing Cultures of Aerobic and Anaerobic Bacteria
Under controlled laboratory conditions, it is possible to adjust oxygen concentrations to maximize the growth rate of a particular bacterial species. Because oxygen diffuses only slowly into liquid, the concentration of oxygen frequently limits the growth rate of aerobic and facultatively anaerobic bacteria in liquid culture. To supply oxygen for the growth of aerobic microorganisms and overcome the growth rate limitations caused by low oxygen concentrations, liquid cultures can be agitated at high speed on a shaker table or by an impeller within the culture vessel, or oxygen can be supplied to the culture vessel through forced aeration (FIG. A). Interrupting the supply of oxygen to an actively growing culture for even a brief period of time can lead to anaerobic conditions, in some cases causing a rapid die-off of the bacteria. Some microbial populations can lose viability if a rotary shaker is turned off for only a few minutes, such as may occur when changing flasks on the shaker table.
Whereas aeration enhances the rates of aerobic growth, oxygen must be excluded from the growth medium to permit the growth of obligate and strict anaerobes. This can be accomplished by adding chemicals that react with and remove molecular oxygen from the growth medium. For example, sodium thioglycollate is frequently added to liquid culture media for the growth of anaerobes because it reacts with molecular oxygen, removing free oxygen from solution.
Similarly, the amino acid cysteine and other compounds containing sulfhydryl groups can also be used to scavenge molecular oxygen from a growth medium. For liquid cultures, nitrogen may be bubbled through the medium to remove air and traces of oxygen, and then the culture vessel is sealed tightly to prevent oxygen from reentering.
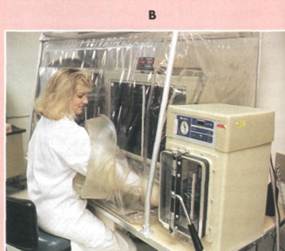
There are many types of anaerobic culture chambers that can be employed to exclude oxygen from the atmosphere (FIG. B). Common forms of anaerobic chambers, such as the Gas Đŕę system, generate hydrogen, which reacts with the oxygen as a catalyst within the chamber to produce water. Carbon dioxide is also generated in this system to replace the volume of gas depleted by the conversion of oxygen to water. It is also possible to combine several approaches to ensure absolute anaerobic conditions. In the Hungate roll tube method, after sterilization of a prereduced medium (a medium from which oxygen is excluded by the incorporation of a chemical that scavenges the free oxygen) within a sealed test tube, the medium is rolled during cooling so that the medium covers the inside of the test tube; the medium is then inoculated with a microorganism under a stream of carbon dioxide or nitrogen and tightly sealed with butyl rubber stoppers to keep oxygen out; the development of microbial colonies can be seen on the tube surface, and individual cultures can be observed without disturbing other cultures.
|

|
A rotary shaker is used to maintain aerobic conditions in liquid cultures.
An anaerobic glove box like this may be used to culture anaerobes.
FACTORS INFLUENCING BACTERIAL GROWTH 303
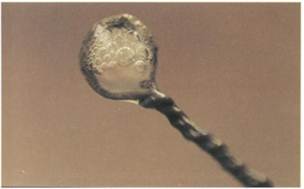
FIG. 10-14 The action of catalase is readily visualized when hydrogen peroxide is added to cells that have produced this enzyme.
intermediary form of oxygen known as the superoxide anion (02"), in addition to forming singlet oxygen. The superoxide anion is converted to hydrogen peroxide and oxygen by the action of the enzyme superoxide dismutase, which is produced by most aerobic and facultatively anaerobic bacteria. Superoxide dismutase removes the toxic superoxide anion but forms hydrogen peroxide (H202), which is also toxic. The reaction that describes the action of superoxide dismutase is:
Hydrogen peroxide is frequently used to kill bacteria, for example, when hydrogen peroxide is applied to a cut to prevent infection. Some bacteria produce enzymes that destroy hydrogen peroxide. Catalase converts hydrogen peroxide to water and oxygen. The reaction that describes the action of Cata-
Obligate aerobes and facultative anaerobes usually produce both catalase and superoxide dismutase (Table 10-3). These enzymes permit such microorganisms to grow without accumulating toxic forms of oxygen. In contrast, obligate anaerobes, such as Clostridium species, generally lack these enzymes. The inability of these organisms enzymatically to remove toxic forms of oxygen probably accounts for the fact that they are obligately anaerobic and sensitive to oxygen.
Salinity
Halophiles are bacteria that specifically require sodium chloride for growth (FIG. 10-15). Moderate halophiles, which include many marine bacteria, grow best at salt (NaCl) concentrations of about 3% NaCl. Extreme halophiles exhibit maximal growth rates in saturated brine solutions. These organisms grow quite well in salt concentrations of greater than 15% NaCl and can grow in places like salt lakes and pickle barrels (FIG. 10-16). High salt concentrations normally disrupt membrane transport systems and denature proteins. Extreme halophiles must possess physiological mechanisms for tolerating high salt concentrations. For example, Halobacterium, possesses an unusual plasma membrane and many unusual enzymes that require a high salt concentration for activity.
Halophiles require a high salt concentration for growth.
Most bacteria, however, do not possess these physiological adaptations and cannot tolerate high
Bacterial production of catalase can be demonstrated by adding a loopful of a microbial culture to a 3% solution of hydrogen peroxide. The evolution of gas bubbles, oxygen, is evidence of the action of the catalase (FIG. 10-14).
Date: 2015-02-28; view: 2122
| <== previous page | | | next page ==> |
| Deep Sea Thermal Vent Bacteria—cont'd | | | FACTORS INFLUENCING BACTERIAL GROWTH 305 |