
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
PAVED WITH TITANIUM
The streets of London may soon be paved with a rare metal, but instead of the gold of fairy tales, it will be titanium and will serve to absorb pollution caused by car exhausts. The new 'Noxer' paving blocks, pioneered by Japan's Mitsubishi Materials Corporation, feature a 5-7 mm thick surface layer of titanium (IV) oxide (titanium dioxide) on a cement mortar. Titanium (IV) oxide (Figure 1) is a photocatalyst, which uses sunlight to absorb and render harmless oxides of nitrogen (NO and NO2, or NO ) emitted from passing traffic — a principal cause of air pollution. The chemical reaction is said to work in both wet and dry conditions and should help reduce urban pollution levels.

Fig. 1 The structure of titanium (IV) oxide (titanium dioxide).
NOx gases are produced whenever fuel is burned, with road traffic being the biggest producer of NOX in the UK. In sunlight, NOX emissions can react with hydrocarbons to produce a variety of harmful substances called photochemical oxidants. These include a-oxoethylperoxylnitrate (peroxy acetyl nitrate, or PAN) and ozone, both of which are irritating to humans and cause damage to plants. In addition to reacting with hydrocarbons, NOX emissions are further oxidised in the atmosphere, contributing to the production of acid rain. Additionally, there is global concern that an increase in these gases, of which some are strong infrared absorbers, may alter the radiative balance of the atmosphere (the heat balance resulting from the equilibrium between incoming radiation from the sun and outgoing radiation from the Earth), leading to climate change.
When a titanium dioxide crystal is exposed to the ultraviolet rays in sunlight, it absorbs light and electrons are excited. The movement of excited electrons leads to the generation of active oxygen (also known as excited oxygen or free radical oxygen). Active oxygen has a high oxidation efficiency, meaning that the titanium (IV) oxide has a strong ability to oxidise various substances that are sticking to the surface. For example, NOX from the air is oxidised into nitrate ions (NO3~). This photocatalytic reaction shows a stronger oxidative power than that of most other metal-based catalysts. The resultant nitrate is then washed away by rain, or permeates the block to form stable nitrate compounds within the concrete. Figure 2 shows the basic principle behind the function of the Noxer blocks, in both wet and dry weather conditions.
The City of Westminster in the heart of London, on whose 400 miles of pavement can be found Big Ben and the Houses of Parliament, will become the first place in Europe to test the slabs, with trials planned for the near future. If the experiment is successful, the council will begin repaving selected footways over the next few years. Paving on the streets of Westminster is renewed at a rate of around 10 to 20 miles a year, so the new stones could be laid in place of the usual paving. In Japan, where concerns about illness as a result of car emission gases are increasing, the stones have replaced ordinary paving blocks in around 30 towns across the country, since being originally tested in Osaka in 1997.
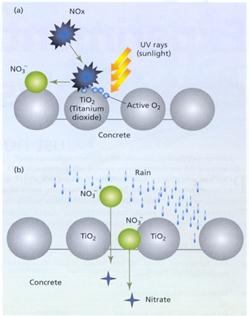 Fig. 2
Fig. 2
(a) Sunlight enables a TiO2 coated Noxer block to eliminate NOX from its surface by converting the NOX to nitrate (NO3~).
(b) When it rains the nitrate that permeates the block forms stable nitrate compounds within the concrete.
Currently, around 75% of the UK population lives within urban areas, and poor air quality contributes substantially to ill health in this country. Although the slabs do not remove all the harmful fumes, they are an innovative 'step' in a more environmentally friendly direction.
Windows that clean themselves
Early in 2001, international glass manufacturers Pilkington launched a revolutionary new product that could mean an end to the traditional 'sponge-and-bucket' method of cleaning external windows. Pilkington Activ™ is the first glass of its kind to possess the ability to clean itself. This may not be a popular breakthrough with window cleaners, but it does offer several benefits. These include the saving of cleaning time for residential and commercial windows, and a reduction in expenditure on cleaning products. Self-cleaning windows will reduce the need for ladders and industrial cleaning equipment that must be operated at extreme heights. On an environmental upside, the glass reduces the run-off of potentially harmful detergents.
The self-cleaning glass uses the ultraviolet rays of the Sun to steadily and continuously break down and dissolve organic dirt in a photocatalytic reaction, while simultaneously reducing the surface tension of water, causing it to spread out and 'sheet' down the surface of the glass and wash away dirt.
The Sun...
A microscopically thin, transparent coating of titanium (IV) oxide (titanium dioxide) gives the glass its special properties. This patented coating is bonded permanently to the surface of the glass while it is in its molten state. This means that the composition becomes part of the surface, rather than simply a coating. The overall thickness of the active coating is around 50nm (50 millionths of a millimetre — the comparative equivalent of the thickness of a penny coin on top of the Empire State Building).
The photocatalytic effect (Figure 1) arises because titanium dioxide can absorb the ultraviolet component of natural sunlight, causing its electrons to become excited (move to a higher energy level). These electrons react with oxygen in the air to produce free radical oxygen. The active oxygen molecules, no longer in their balanced electronic state, are powerful oxidants, capable of attacking organic dirt on the glass. These reactions clean the window by breaking down the organic material to form mainly carbon dioxide and water.
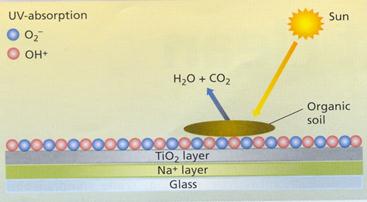
Figure 1. The photocatalytic effect. The coating's photoactivity breaks down organic material, reducing the adherence of dirt to the surface.
...and the rain
The hydrophilic properties of titanium dioxide also add to the cleaning effect. On regular glass, rainfall tends to form droplets on the surface, typically 3-4 mm in diameter. These droplets aggregate together and, when they reach a certain mass, flow down the glass in small streams (Figure 2). This concentrates dirt on the window pane, resulting in smears, smudges and droplet drying marks. The titanium dioxide coating has hydroxylated groups at the surface. These alter droplet formations by changing the glass surface from hydrophobic to hydrophilic. The titanium dioxide forms strong hydrogen bonds with the water, overcoming the effects of surface tension. This means that 'droplets' are not formed on the coating because the water spreads out into an almost uniform film on the surface. On Pilkington Activ™ a single droplet of water can cover an area of over 4cm2 compared to less than 0.2cm2 on regular glass.

Figure 2 Comparison of Pilkington Activ™ and regular float glass in external windows.
Date: 2015-01-29; view: 4498
| <== previous page | | | next page ==> |
| A REVIEW OF HOW NANOTECHNOLOGY RELATES TO VARIOUS DISCIPLINES | | | COMING TO A WINDOW NEAR YOU |