
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Plant, Animal and Bacterial Cells
When the individual cells of plants and animals (Fig. 1.3) are compared, they have many characteristics in common. Cells are the basic structural and functional units of both plants and animals. They share many characteristics: both are multicellular organisms; all plant and animal cells are eukaryotic; and most organelles are present in both types of cells. Some characteristics, however, are unique to the cells of each type of an organism.
| Fluid-filled pinocytic vesicle Centriole Small vacuole Smooth endoplasmic reticulum Lysosome | 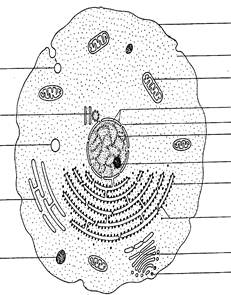
| Cell membrane Cytoplasm Mitochondrion Nuclear membrane Pore in membrane Nucleus Nucleolus Rough endoplasmic reticulum Ribosomes Golgi apparatus Secretory vesicle |
| Fig. 1.3. Ultrastructure of generalised animal cell |
Plant cells may contain three structures not found in animal cells: cell walls, large central vacuoles, and plastids are characteristic. Centrioles are found in some but not all types of plant and animal cells. Bacteria and blue-green bacteria are quite different from other cells. They have fewer structures than plant or animal cells; they carry out all of the life processes that other cells carry out. A bacterium has a cell wall, a cell membrane and cytoplasm. But, there is no nucleus, nuclear membrane, endoplasmic reticulum or mitochondrium. The chromosome material, which directs the cell’s activities, floats freely through the cytoplasm.
1.2. Energy and Energy Conversions. Chemistry aspects of life organisation
Physicists define energy as the capacity to do work which occurs when a force operates on an object over a distance. In biochemistry, it is more useful to consider energy as the capacity for change. No cell creates energy—all living things must obtain energy from the environment. Indeed, one of the fundamental physical laws is that energy can neither be created nor destroyed. However, energy can be transformed from one type into another and living cells carry out many such energy transformations. Energy transformations are linked to the chemical transformations that occur in cells—the breaking of chemical bonds, the movement of substances across membranes and so forth. Energy changes are related to changes in matter. Energy comes in many forms, such as chemical energy, light energy and mechanical energy. But all forms of energy can be considered as one of two basic types:
Kinetic energy is the energy of movement. This type of energy does work that alters the state or motion of matter. It can exist in the form of heat, light, electric energy and mechanical energy among others.
Potential energy is the energy of state or position—that is, stored energy. It can be stored in chemical bonds, as a concentration gradient and as electric potential among other ways. Water stored behind a dam has potential energy. When the water is released from the dam, some of this potential energy is converted into kinetic energy which can be harnessed to do work.
Likewise, fatty acids store chemical energy in their C—H bonds and C—C bonds and that energy can be released to do biochemical work. Most of living beings consist of cells. In all cells of all organisms, two types of metabolic reactions occur:
Anabolic reactions (anabolism) link together simple molecules to form more complex molecules. The synthesis of a protein from amino acids is an anabolic reaction. Anabolic reactions require an input of energy and capture it in the chemical bonds that are formed.
Catabolic reactions (catabolism) break down complex molecules into simpler ones and release the energy stored in chemical bonds.
Catabolic and anabolic reactions are often linked. The energy released in catabolic reactions is used to drive anabolic reactions—that is, to do biological work. Cellular activities such as growth, movement and active transport of ions across a membrane all require energy and none of them would proceed without a source of energy. In the discussion that follows, will be discovered the physical laws that govern all energy transformations, identified the energy available to do biological work and considered the direction of energy flow. All these processes can be explained by laws of thermodynamics.
The first law: Energy is neither created nor destroyed
Energy can be converted from one form to another. For example, by striking a match, you convert potential chemical energy to light and heat. The first law of thermodynamics states that in any such conversion of energy, it is neither created nor destroyed.
The first law tells us that in any conversion of energy from one form to another, the total energy before and after the conversion is the same. Potential energy in the chemical bonds of carbohydrates and lipids can be converted to potential energy in the form of ATP. This energy can then be used to produce potential energy in the form of concentration gradients established by active transport and can be converted to kinetic energy and used to do mechanical work, such as muscle contraction.
The second law: Not all energy can be used and disorder tends to increase
The second law of thermodynamics states that, although energy cannot be created or destroyed, when energy is converted from one form to another, some of that energy becomes unavailable to do work. In other words, no physical process or chemical reaction is 100 percent efficient and not all the energy released can be converted to work. Some energy is lost to a form associated with disorder. The second law applies to all energy transformations but we will focus here on chemical reactions in living systems.
Not all energy can be used
In any system, the total energy includes the usable energy that can do work and the unusable energy that is lost to disorder: total energy = usable energy + unusable energy.
In biological systems, the total energy is called enthalpy (H). The usable energy that can do work is called free energy (G). Free energy is what cells require for all the chemical reactions of cell growth, cell division and the maintenance of cell health. The unusable energy is represented by entropy (S) which is a measure of the disorder of the system multiplied by the absolute temperature (T). Thus, it is possible to rewrite the word equation above more precisely as H = G + TS,
Or, if rearrange this expression, G = H – TS (usable energy).
Although G, H or S cannot be measured absolutely, it is possible to determine the change in each at a constant temperature. Such energy changes are measured in calories (cal) or joules (J). A change in energy is represented by the Greek letter delta (D). For example, the change in free energy (DG) of any chemical reaction is equal to the difference in free energy between the products and the reactants, DGreaction = Gproducts – Greactants.
If the necessary free energy is not available, the reaction does not occur.
Depending on the sign and magnitude of DS, the entire term, T x S, may be negative or positive, large or small. In other words, in living systems at a constant temperature (no change in T), the magnitude and sign of DG can depend a lot on changes in entropy. Large changes in entropy make DG more negative in value, as it is shown by the negative sign in front of the T x S term. If a chemical reaction increases entropy, its products are more disordered or random than its reactants. If there are more products than reactants, as in the hydrolysis of a protein to its amino acids, the products have considerable freedom to move around. The disorder in a solution of amino acids will be large, compared with that in the protein in which peptide bonds and other forces prevent free movement. Thus, in hydrolysis, the change in entropy (DS) will be positive. If there are fewer products and they are more restrained in their movements than the reactants, DS will be negative.
For example, a large protein linked by peptide bonds is less free in its movements than a solution of the hundreds or thousands of amino acids from which it was synthesized.
Disorder tends to increase
The second law of thermodynamics also predicts that, as a result of energy conversions, disorder tends to increase. Chemical changes, physical changes and biological processes all tend to increase entropy and therefore tend toward disorder or randomness. This tendency for disorder to increase gives a directionality to physical processes and chemical reactions. It explains why some reactions proceed in one direction rather than another. How does the second law apply to organisms? Consider the human body with its highly complex structures constructed of simpler molecules. This increase in complexity is in apparent disagreement with the second law. But this is not the case. Constructing 1 kg of a human body requires that about 10 kg of biological materials be metabolized and in the process converted to CO2, H2O and other simple molecules, and these conversions require a lot of energy. This metabolism creates far more disorder than the order in 1 kg of flesh.
Life requires a constant input of energy to maintain order
There is no disagreement with the second law of thermodynamics. Having seen that the physical laws of energy apply to living things, it will be now considered how these laws apply to biochemical reactions.
Chemical reactions release or take up energy
In cells, anabolic reactions may make a single product, such as a protein (a highly ordered substance), out of many smaller reactants, such as amino acids (less ordered). Such reactions require or consume energy. Catabolic reactions may break down an ordered reactant, such as a glucose molecule, into smaller, more randomly distributed products, such as carbon dioxide and water. Such reactions give off energy. In other words, some reactions release free energy and others take it up. The amount of energy released (–DG) or taken up (+DG) by a reaction is related directly to the tendency of the reaction to run to completion (the point at which all the reactants are converted to products). Some reactions tend to run toward completion without any input of energy. These reactions, which release free energy, are said to be exergonic and have a negative DG. Reactions that proceed toward completion only with the addition of free energy from the environment are endergonic and have a positive DG. If a reaction runs exergonically in one direction (from reactant A to product B, for example), then the reverse reaction (B to A) requires a steady supply of energy to drive it. If A→B is exergonic (DG < 0), then B →A is endergonic (DG > 0). In principle, chemical reactions can run both forward and backward. For example, if a compound A can be converted into a compound B (A→B), then B, in principle, can be converted into A (B →A), although in given concentrations of A and B, only one of these directions will be favored. Think of the overall reaction as resulting from competition between forward and reverse reactions (A~B). Increasing the concentration of the reactants (A) speeds up the forward reaction and increasing the concentration of the products (B) favors the reverse reaction. At some concentration of A and B, the forward and reverse reactions take place at the same rate. At this concentration, no further net change in the system is observable, although individual molecules are still forming and breaking apart. This balance between forward and reverse reactions is known as chemical equilibrium. Chemical equilibrium is a static state, a state of no net change and a state in which DG = 0.
There is good reason to believe that life, as it is known to be, cannot exist without water. Animals and plants that live on the Earth’s land masses had to evolve elaborate ways to retain the water that makes up about 70 percent of their bodies. Aquatic organisms living in water do not need these water-retention mechanisms; thus, biologists have concluded that the first living things originated in a watery environment. This environment need not have been the lakes, rivers and oceans with which we are familiar. Living organisms have been found in hot springs at temperatures above the usual boiling point of water, in a lake beneath the frozen Antarctic ice, in water trapped 2 miles below the Earth’s surface, in water 3 miles below the surface of the sea, in extremely acid and extremely salty water, and even in the water that cools the interiors of nuclear reactors. With 20 trillion galaxies in the universe, each with 100 billion stars, there are many planets out there and if our own solar system is typical, some of them have the water needed for life. As biologists contemplate how life could originate from nonliving matter, their attention focuses not just on the presence of water but on what is dissolved in it. A major discovery of biology is that living things are composed of the same types of chemical elements as the vast nonliving portion of the universe. This mechanistic view— that life is chemically based and obeys universal physicochemical laws—is a relatively recent one in human history. The concept of a “vital force” responsible for life, different from the forces found in physics and chemistry, was common in Western culture until the nineteenth century and many people still assume such a force exists. However, most scientists adhere to a mechanistic view of life and it is the cornerstone of medicine and agriculture. Before describing how chemical elements are arranged in living creatures, some fundamental chemical concepts on changes of matter will be considered. In addition to changes in state (solid to liquid to gas), substances undergo changes that transform both their composition and their characteristic properties.
The first chemical signatures indicating the presence of life here are about 4 billion years old. Thus, it took 600 million years, during a geological time frame called the Hadean, for the chemical conditions on Earth to become just right for life. Key among those conditions was the presence of water. The Ancient Earth probably had a lot of water high in the atmosphere. But the new planet was hot and this water evaporated into space. As the Earth cooled, it became possible for water to remain on its surface but where did that water come from? One current view is that comets—loose agglomerations of dust and ice that have orbited the sun since the planets formed—struck the Earth repeatedly and brought not only water but other chemical components of life, such as nitrogen. As the Earth cooled, chemicals from the rocks dissolved in the water and simple chemical reactions took place. Some of these reactions could have led to life but impacts of large comets and rocky meteorites would have released enough energy to heat the developing oceans almost to boiling, thus destroying any early life. These large impacts eventually subsided and life gained a foothold about 3.8 to 4 billion years ago. The prebiotic Hadean was over. The Archean had begun and there has been life on the Earth ever since. Like the rest of the chemical world, living things are made up of atoms and molecules.
Date: 2014-12-22; view: 3622
| <== previous page | | | next page ==> |
| Cells Vary in Shape, Size and Arrangement | | | Life and Chemistry: Atoms and Small Molecules |