
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Most Important Oxidizing and Reducing Agents
What substances can display the properties of oxidizing agents, and what - of reducing agents? We have already mentioned that an oxidizing agent contains an element whose oxidation number decreases, while a reducing agent contains an element whose oxidation number grows in the course of a reaction. Consequently, oxidizing agents will include first of all compounds with the higher, and reducing agents will include compounds with the lower oxidation numbers featuring a given element.
Metals display only a positive oxidation state in their compounds, and their minimum oxidation number is zero. In other words, they have the minimum oxidation number only in the free state. Indeed, all free metals, although to a different extent, are capable of exhibiting only reducing properties. The reducing agents used in practice include Aluminium, Magnesium, Sodium, Potassium and Zinc. If a metal can have several oxidation numbers, those of its compounds in which it displays the lowest of them are also reducing agents, as a rule. Examples are the compounds of Iron (II), Tin (II), Chromium (II) and Copper (I).
Those compounds of metals can be oxidizing agents in which the oxidation number is high and either is equal to the number of the group, which the metal belongs to or is close to it. Practical use has been found, in particular, by an Ammonia solution of Silver oxide, an Ammonia solution of Copper (II) Sulfate, Mercury (II) Chloride, Lead (IV) Oxide, Iron (III) Chloride, Potassium Chromate and Dichromate (K2CrO4 and K2Cr2O7), Potassium Permanganate KMnO4, and Manganese (IV) oxide MnO2.
Non-metals exhibit both positive and negative oxidation states. It is natural that compounds containing non-metals in their higher positive oxidation states can be oxidizing agents, and compounds in which a non-metal displays a negative oxidation state can be reducing agents.
The most important reducing agents are Hydrogen H2, Carbon C and Carbon (II) Oxide CO.
Non-metals of the upper part of groups VI and VII of the Periodic Table are strong oxidizing agents. The strong oxidizing properties of these substances are explained by the high electronegativity of their atoms. Fluorine F2 has the strongest oxidizing properties, but in practice Oxygen O2, Chlorine Cl2 and Bromine Br2 are used most frequently as oxidizing agents.
The compounds used as oxidizing agents also include acids. Hydrochloric HCl, Sulfuric H2SO4 and nitric HNO3 acids have the greatest practical significance. The oxidizing element in Hydrochloric acid is Hydrogen H+; in Nitric acid it is Nitrogen N5+, in dilute Sulfuric acid - Hydrogen H+, and in the concentrated acid - Sulfur S+6. Hence, the equation of reduction with Hydrochloric and dilute Sulfuric and a few other acids (H3PO4, CH3COOH, HClO4) has the form:
2H+ + 2  → H2.
→ H2.
Nitric acid, depending on its concentration, temperature, and the nature of the reducing agent, can be reduced to different oxidation numbers of the Nitrogen. One of the usual products of its reduction is Nitrogen (II) Oxide NO:
NO3_ + 4H+ + 3  = NO + 2 H2O.
= NO + 2 H2O.
Various products may also be formed in reduction with concentrated Sulfuric acid. One of them is Sulfur (IV) Oxide:
SO42- + 4H+ + 2  = SO2 + 2H2O.
= SO2 + 2H2O.
Other compounds of non-metals used as oxidizing agents are Hydrogen Peroxide H2O2, the salts of acids in which the acid-forming element exhibits a high oxidation number - Chlorates (KClO3), Perchlorates (KClO4).
Possible oxidation numbers of some chemical elements are presented in Appendix 10.
5. Types of Redox Reactions
In all reactions, presented in sub-chapter 3, oxidizing and reducing atoms are parts of different substances. Such type of redox reactions is the most extended and called Intermolecular Oxidation-Reduction.
Compounds with the maximum oxidation number of a given element can play the role of oxidizing agents in redox reactions. The oxidation number can only be lower in this case. Conversely, compounds with the minimum oxidation number can only be reducing' agents. Here the oxidation number of an element can only grow. If an element is in an intermediate oxidation state, however, its atoms can, depending on the conditions prevailing, either take on or give up electrons. In the first case, the oxidation number of the element will be lower, and in the second one it will grow. Consequently, compounds containing elements in intermediate oxidation states have oxidation-reduction duality - they are capable of entering into-reactions with either oxidizing or reducing agents.
For example, Nitrogen forms compounds in which its oxidation number changes from -3 (Ammonia and Ammonium salts) to +5 (Nitric acid and its salts). The Nitrogen in Ammonia can only be a reducing agent, and that in Nitric acid - only an oxidizing agent. Nitrous acid HNO2 and its salts, in which the oxidation number of Nitrogen is +3 however, enter into reactions with both strong oxidizing and strong reducing agents. In the first case, it is oxidized to Nitric acid, and in the second, it is usually reduced to Nitrogen (II) Oxide NO. We can exemplify the oxidation-reduction duality of Nitrous acid and its salts by the reactions:
5 KNO2 + 2 KMnO4 + 3 H2SO4 = 5 KNO3 + 2 MnSO4 + K2SO3 + 3 H2O -
N+3 is a reducing agent;
2 HNO2 + H2S = 2 NO + S + 2H2O -
N+3 is an oxidizing agent.
In addition to Nitrous acid HNO2, Sulfur S, Iodine I2, Hydrogen Peroxide H2O2 and a number of other substances have oxidation-reduction duality.
Substances containing element in intermediate oxidation state often have another characteristic property. In definite conditions one part of the element is oxidized and the other part is reduced. This process is known as autoxidation-autoreduction. For instance, when Chlorine reacts with water, the mixture of Hydrochloric and Hypochlorous acids is produced:
Cl2 + H2O = HCl + HClO. 
Here the Chlorine undergoes both oxidation and reduction:
Cl2 + 2  = 2 Cl- = 2 Cl-
| 1 (reduction); |
Cl2 - 2  = 2Cl+ = 2Cl+
| 1 (oxidation). |
Autoxidation-autoreduction is also called disproportionation.
Some compounds in definite conditions (usually when heated) experience intramolecular oxidation-reduction. In this process, one constituent part of the substance is an oxidizing agent, and the other is a reducing one. Examples of intramolecular oxidation-reduction are many processes of thermal dissociation. For instance, when Potassium Chlorate decomposes by heating:
2 KClO3 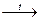 2 KCl + 3 O2
2 KCl + 3 O2
the Oxygen is oxidized (its oxidation number grows from -2 to 0), and the Chlorine is reduced (its oxidation number diminishes from +5 to -1).
Another example is the decomposition of Ammonium Nitrite employed in the laboratory to obtain pure Nitrogen:
NH4NO2 = N2 + 2H2O.
Here the ion NH3 is oxidized and the ion NO2- is reduced to free Nitrogen.
Date: 2015-01-12; view: 4292
| <== previous page | | | next page ==> |
| Compiling Equations of Oxidation-Reduction Reactions | | | Influence of Medium to Redox Reactions |