
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
PRACTICE PROBLEMS
1. What are the three major types of bonds?
2. What is the role of electronegativity in the determination of the ionic or covalent character of a bond?
3. What type of bond would be expected between H and F; Cu and S; Br and I?
4. List the three bond pairs referred to in the previous question in order to increase ionic characters.
—HAPTER # 5. LABORATORY GLASSWARE, LABWARE AND RULES OF LABORATORY RESEARCH
The most useful chemical glassware is presented in Fig. 12 and 13.

 Figure 12. Chemical Glassware
Figure 12. Chemical Glassware


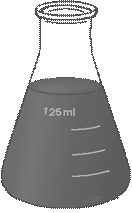

| Test tube | Beaker | Erlenmeyer Flask | Florence Flask |
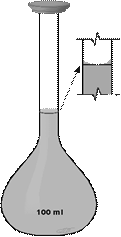


| Volumetric Flask | Dropper Bottle | Glass Jar | Filtering Funnel |




| Buchnerís Funnel | Goochís Crucible | Walterís Crucible Holder | Pi-Pump |


| Graduated Cylinder | Test tube clamp | Clamps |


| Watch glass | Thermometers |

Spatulas
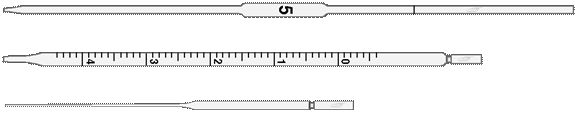
Volumetric pipettes



| Evaporation dish | Pestle and mortar | Forceps |

Crystallizing Dish as sand bath
Figure 13. Separate varieties of laboratory glassware
1. Chemical glassware
All chemical glassware is divided into several groups according to their use:
Ø of general usage (is used to carry out different chemical operations): test tubes, beakers, flat-bottom, round-bottom and conical flasks, Wurtz flasks (round-bottom with bleeder), crystallizer tanks, funnels, clock glass, weighing bottles;
Ø measuring vessels: graduated cylinders, measuring tubes, measuring pipettes, volumetric flasks;
Ø of special use: flasks, Kipp gas generator, vacuum filtering apparatus, consisting of Bunsen flask, Buchner funnel, trap flask, and water-jet pump;
Ø porcelain ware and ware made from other materials: spreading rods, mortars, evaporating dishes, crucibles and boats.
Date: 2015-01-12; view: 1460