
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Theories of atomic structure
Historically development of ideas about atomic structure passed some stages:
Ø Concept of G.G. Thompson (sometimes named theory of “pudding with raisins”). Practically it was the first scientific theory, which described an atom as continuous positive charged continuum of nucleus with dissemination of little negatively charged electrons. This theory was destroyed after the experiments made by Sir Reserford using α-particles (nuclear of helium) bombed metallic foils. It was determined that the most of particles passed over foils without any changes. It means that nucleus is not a continuous continuum.
Ø Reserford’s theory was based on the idea that atomic structure is similar to Solar system (another name of this theory is “planetary”). According to this theory atom consists of massive compact nucleus and electrons turned around it like planets around the Sun. But this idea mismatched to the concepts of classic electrodynamics (Maxwell’s theory). In accordance with one moving electron must loss energy uninterruptedly and finally fall on nuclear. But in reality atoms and all world exist without serious problems. Danish outstanding scientist N. Bore improved planetary theory using some concepts called postulates of Bore. He proposed that electron does not radiate energy when moving on so-called “stable” orbits. Energy can radiates or absorbs when electron jumps from one orbit to another by separated portion - quantum of energy.
| |
|
 But it turned out, that Bore’s theory is valid absolutely only for atom of Hydrogen. The cause of this is in mechanistic approach to the nature of electron. Electron according to Bore’s theory is like a very small boll. But further development of natural science in particular Einstein’s research shows that micro-world have other laws than macro-world.
But it turned out, that Bore’s theory is valid absolutely only for atom of Hydrogen. The cause of this is in mechanistic approach to the nature of electron. Electron according to Bore’s theory is like a very small boll. But further development of natural science in particular Einstein’s research shows that micro-world have other laws than macro-world.
Ø Modern theory proceeds from the assumption that electron combines the property of physical body and electromagnetic wave. And therefore it is necessary to use other notion for describing of electron moving around nucleus – electronic orbital. Electronic orbital is the part of space where the probability of electron being is 90% or where electron spends 90% of time. Schematically electronic orbital can be described by Fig. 2:
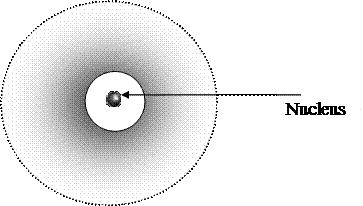 |
Date: 2015-01-12; view: 1559
| <== previous page | | | next page ==> |
| Types of chemical reactions | | | Figure 2. Scheme of Electron orbital |