
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
EXPERIMENTAL PART
1. Dissociation of weak electrolytes
a) Fill two test tubes with 3-4 drops of diluted acetic acid and add 1 drop of an indicator (methylorange). Add some solid sodium acetate into one of the test tubes. Compare colors of the solutions in the test tubes. Explain the results of the experiment.
b) Repeat the experiment with the solution of ammonium hydroxide. Use phenolphtalein as indicator and add ammonium chloride.
2. Formation of weak electrolytes
a) Take 3-4 drops of a CH3COONa solution in a test tube and add some diluted HCl. Check the smell before and after the experiment. Explain the results of the experiment.
b) Repeat the experiment with NH4Cl and NaOH respectively.
3. Amphoteric hydroxides
a) Take 5-6 drops of a solution of any chromium (III) salt and add dropwise a diluted solution of sodium hydroxide until the precipitate of chromium hydroxide is formed. Divide the precipitate of Cr(OH)3 into two parts and dissolve them in NaOH and HCl respectively.
b) Repeat the same experiment using any zinc salt.
QUESTIONS AND PROBLEMS
1. The dissociating constant of butyric acid C3H7COOH is 1.5 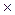 10
10 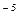 . Calculate the degree of its dissociation in a 0.005 M solution.
. Calculate the degree of its dissociation in a 0.005 M solution.
2. What is the hydrogen ion concentration [H+] in an aqueous solution of formic acid if a = 0.03?
3. Calculate the concentration of acetate ions in 0.1 M solution of acetic acid in presence of 0.01 M HCl.
4. Calculate the ionic strength and the activities of the ions in a solution containing 0.01 mole×l-1 of Ca(NO3)2 and 0.01 mol/l of CaCl2.
Experiment 2
Date: 2015-01-12; view: 1274
| <== previous page | | | next page ==> |
| QUESTIONS AND PROBLEMS | | | HETEROGENOUS EQUILIBRIUM |