
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
QUESTIONS AND PROBLEMS
1. Find the value of the rate constant for the reaction A + B 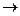 AB if at concentration of substances A and B equal to 0.05 M and 0.01 M respectively, the rate of the chemical reaction is 5 x 10
AB if at concentration of substances A and B equal to 0.05 M and 0.01 M respectively, the rate of the chemical reaction is 5 x 10 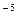 M/min.
M/min.
2. How many times will the rate of the reaction 2A + B 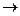 A2B change if the concentration of A is doubled, and that of B is halved ?
A2B change if the concentration of A is doubled, and that of B is halved ?
3. What is the temperature coefficient of the reaction rate if the rate grows 15.6 times when the temperature is increased by 30 Kelvins?
4. Find the equilibrium constant of the reaction N2O4  2NO2 if the initial concentration of the N2O4 was 0.08 M, and by the moment when equilibrium was established 50% of N2O4 was dissociated.
2NO2 if the initial concentration of the N2O4 was 0.08 M, and by the moment when equilibrium was established 50% of N2O4 was dissociated.
5. In which direction will the following equilibria shift :
a) 2CO + O2  2CO2
2CO2 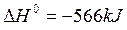
b) N2 + O2  2NO
2NO 
( 1 ) when the temperature is lowered; ( 2 ) when the pressure is increased; ( 3 ) when the concentration of oxygen is increased ?
Experiment 1
IONIC EQUILIBRIUM
Some substances while being dissolved interact with molecules of a solvent. As a result they dissociate and form ions. The process of dissociation can be written as:
AnBm 
 nAm+ + mB
nAm+ + mB 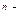
Na2CO3  2Na+ + CO
2Na+ + CO 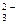
Ca(OH)2  Ca2+ + 20H
Ca2+ + 20H 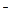
The process of dissociation can be quantitatively characterized by a degree of dissociation (a) and a dissociation constant (Kdis).
a = 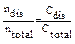
n - number of molecules (moles)
C - concentration
If a > 0.3, the electrolyte is called strong. Strong electrolytes are salts, strong acids (HCl, H2SO4, HNO3, HClO4 and some others), hydroxides of alkaline and alkaline-earth metals.
If a < 0.03, the electrolyte is called weak. The dissociation of a weak electrolyte is a reversible and a stepwise process.
Kdis is determined as an equilibrium constant of a reaction of dissociation of an electrolyte.
H3PO4  H+ + H2PO
H+ + H2PO 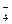
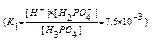
H2PO4-  H+ + HPO
H+ + HPO 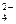

HPO 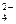
 H+ + PO
H+ + PO 
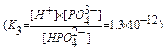
K1,K2,K3 - stepwise dissociation constants.
For the overall process of the dissociation of phosphoric acid

The dissociation constant of an electrolyte doesn't depend on concentration but increases with the evaluation of temperature.
For an electrolyte AB dissociating into ions A+ and B 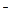 the dissociation constant and the degree of dissociation are related by the equation (Ostwald's dilution law):
the dissociation constant and the degree of dissociation are related by the equation (Ostwald's dilution law):
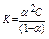
For very weak electrolytes and very diluted solutions the equation may be simplified:
K = a2C
If an ion common to one of the ions formed in the dissociation process of a weak electrolyte is introduced into the solution, equilibrium of dissociation is violated and shifts towards the direction of formation of undissociated molecules so that the degree of dissociation of the electrolyte diminishes. For instance, the addition of ammonium ions (for example ammonium chloride) to a solution of ammonium hydroxide leads to an increase in concentration of NH4+ ions and, in accordance with Le Chatelier's principle, the equilibrium of the dissociation NH4OH  NH4+ + OH
NH4+ + OH 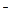 shifts to the left.
shifts to the left.
Ionic concentrations in solutions of strong electrolytes are quite great so that the forces of interionic interaction manifest themselves appreciably even at low concentration of an electrolyte. As a result, the ions are not completely free. This is why the state of ions in a solution is described, in addition to their concentration, by their activity, i.e. the conditional (effective) concentration of ions in accordance with which they act in chemical reactions.
a = f 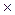 .C
.C
where a is activity; f is activity coefficient
The activity coefficients depend on the composition and concentration of the solution, and on the charge and nature of the ion, and on other conditions. It can be considered approximately that in diluted solutions the activity coefficient of an ion depends only on the charge of the ion and ionic strength of the solution:
log f = - 0.5 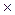 Z2
Z2 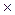 I1/2
I1/2
I = 0.5 (C1 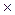 z12 + C2
z12 + C2 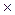 z22 + C3
z22 + C3 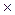 z32 + ... + Ci
z32 + ... + Ci 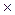 zi2)
zi2)
where z - charge of the ion; I - ionic strength of the solution; C - concentrations of each ion in the solution
Equilibria in solutions of amphotheric electrolytes
Such hydroxides as Al(OH)3, Cr(OH)3, Zn(OH)2, Pb(OH)2, Sn(OH)2 and some other can form both H+ and OH 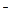 ions while dissociating in saturated solutions:
ions while dissociating in saturated solutions:
2H2O + Zn2+ + 2OH 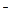
 Zn(OH)2
Zn(OH)2 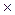 2H2O
2H2O  H2[Zn(OH)4]
H2[Zn(OH)4]  2H+ + Zn(OH)4]2
2H+ + Zn(OH)4]2 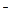
3H2O + Al3+ + 30H 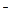
 Al(OH)3
Al(OH)3 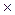 3H2O
3H2O  H3[Al(OH)6]
H3[Al(OH)6]  3H+ + Al(OH)6]
3H+ + Al(OH)6] 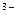
The addition of strong acids and bases shifts the equilibria of the dissociation process so that only one reaction becomes possible.
Date: 2015-01-12; view: 1386
| <== previous page | | | next page ==> |
| RATE OF A CHEMICAL REACTION. CHEMICAL EQUILIBRIUM | | | EXPERIMENTAL PART |