
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Equipment Cost Factors
Fluid Mechanics, Heat Transfer, and Thermodynamics
Design Project
Production of Acetone
We are investigating the feasibility of constructing a new, grass-roots, 15,000 metric tons/year, acetone plant. As part of the feasibility study, we would like you to investigate some of the details of the feed and reaction sections of the proposed plant.
Acetone Production Reaction
The reaction is given below.
 (1)
(1)
For the purposes of this preliminary evaluation, it is assumed that the reaction occurs in a fluidized bed of catalyst particles. Limitations of this equipment make the maximum possible conversion 90% of the equilibrium conversion; however, practical considerations reduce this value to 80% of the equilibrium conversion.
Feed and Reaction Sections
The PFD for the reaction section is given in Figure 1. Feed to the process consisting of liquid isopropanol, is mixed with recycle liquid isopropanol in V-401. The reactor feed stream must be a vapor at 2.16 bar and 101?C. Stream 3 should have a pressure of approximately 2.3 bar.
The reaction is endothermic, and heat is added to the reactor by a molten salt heated in a furnace, H-401. (Molten salt properties: Cp = 0.373 BTU/lb?F, k = 0.35 BTU/hr ft2?F, m = 1.7 cp, and r = 123 lb/ft3) Exit conditions for the reactor are 350?C and 1.96 bar. Following the reactor, the reaction products are cooled to 20?C. The crude acetone product is obtained by flashing the cooled reactor effluent in V-402. It may be assumed that all hydrogen leaves in the vapor phase. All other components distribute between the vapor and liquid phases.
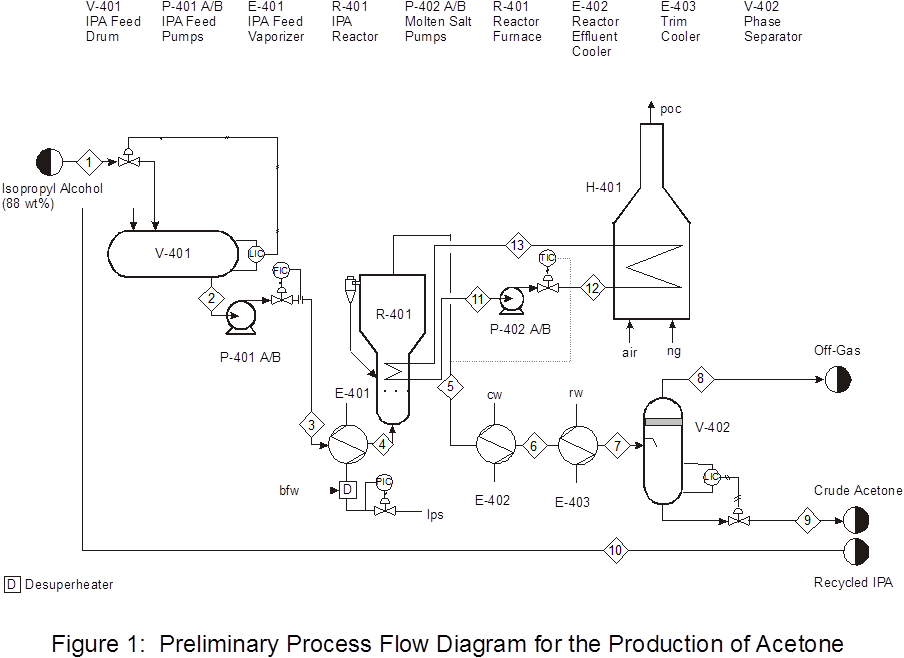
Process Details
Feed Streams
Stream 1: isopropyl alcohol, liquid, 88 wt % IPA, 12 wt % water, 1 atm, 25?C
Stream 10: recycle isopropyl alcohol, assume it contains IPA/water azeotrope at 11% of the molar flow of Stream 1
Effluent Streams
Stream 9: Crude acetone product
Stream 8: Off-gas ? can take credit as fuel gas at lower heating value
Assignment
Your assignment consists of three ?mini-designs.?
1. Optimization of the Feed Section and Molten Salt Loop. (ChE 110) Refer to Figure 1. You are to size P401 A/B and P-402 A/B and determine the optimum pipe sizes for Streams 1-3 and 11-13. Different streams may have different pipe diameters. Isopropanol in Stream 1 is fed from the bottom of a feed tank 20 m away. Pipe runs are at 5 m elevation off the ground, and you should add 90? elbows as necessary. The molten salt loop pipe lengths total 20 m with a 4 m elevation change and eight 90? elbows. Assume that all units are connected by 5 m pipe equivalent length. The objective function is the Equivalent Annual Operating Cost (EAOC) of the feed section and the molten salt loop ($/y). The EAOC is defined as:
 (2)
(2)
where CAP = the installed cost of equipment in the feed section and
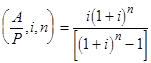 (3)
(3)
where i = 0.15 (15% rate of return) and n = 10 (ten-year plant life).
For the feed section, do not include raw material costs, so CAP includes only the installed cost of pipes and pumps, and operating costs include the electricity to run the pump. For the molten salt loop, do not include the cost of the fired heater or of the molten salt. Specify the liquid level to be maintained in V-401 to avoid cavitation of P-401 A/B. The pump P-401 A/B is 5 m horizontally distant from the V-401 draw. We have a supply of centrifugal pumps used in other plants. Their pump curves and their NPSH curves are attached (Figures 2 and 3). We would like the flexibility for 35% scale-up in the future.
2. Design of E-401.(ChE 111)The heat exchanger, E-401, must be designed in detail. The outlet pressure must be as specified, so the pressure drop in E-401 must be consistent with the pump outlet pressure to achieve the required reactor feed pressure. Note that the outlet stream from this heat exchanger is superheated vapor. If you choose, you may add a desuperheater to reduce the temperature of the steam utility. If so, your choice of temperature must be justified, and the required flowrate of boiler feed water to saturate the steam at the chosen temperature must be calculated.
3. Determination of Conversion Possible in Reactor.(ChE 142) The reactor operates at 1.96 bar and 350?C. You should determine the equilibrium conversion at these conditions and operate at 80% of this conversion.
4. Determination of Break-even Price (BEP) for Crude Acetone.(all classes)A Chemcad simulation for your best case should be presented. You may make any process modifications that improve the BEP, but a full optimization is not required. The break-even price for crude acetone product should be calculated. The best case is defined as the optimum case for each of the ?mini-designs? 1 ? 3, the optimum flash temperature and pressure, plus any other changes to the process that you recommend. For the flash calculation, use Chemcad. You should try at least five different thermodynamics packages and evaluate their prediction of the flash outlet stream conditions. For the final optimization, one package should be chosen and justified. The break-even price for crude acetone product is calculated as follows:
 (4)
(4)
where CAP is now the installed capital cost for the entire feed and reaction sections, including the feed tanks.
 |

Cost Data
Raw Materials
Isopropanol (88 ? 91 wt%) see Chemical Marketing Reporter
Utility Costs
Low Pressure Steam (618 kPa saturated) $6.62/1000 kg
Medium Pressure Steam (1135 kPa saturated) $7.31/1000 kg
High Pressure Steam (4237 kPa saturated) $8.65/1000 kg
Natural Gas (446 kPa, 25C) $3.00/GJ
Fuel Gas $2.75/GJ
use this price for fuel gas credit
Electricity $0.06/kW h
Boiler Feed Water (at 549 kPa, 90C) $2.54/1000 kg
Cooling Water $0.16/GJ
available at 516 kPa and 30C
return pressure 308 kPa
return temperature is no more than 15C above the inlet temperature
Refrigerated Water $1.60/GJ
available at 516 kPa and 10C
return pressure 308 kPa
return temperature is no higher than 20C
Deionized Water $1.00/1000 kg
available at 5 bar and 30?C
Waste Treatment of Off-Gas incinerated - take fuel credit
Equipment Costs (Purchased)
Piping $/m = 5.0 (diameter, in)
Valves $100 (flow diameter, in)0.8
for control valve with orifice plate, double the price
Pumps $630 (power, kW)0.4
Heat Exchangers $1030 (area, m2)0.6
add 25% additional for boilers or evaporators
Compressors $770 (power, kW)0.96+ $400 (power, kW)0.6
assume 70% efficiency
Turbine $2.18´105(power output, MW)0.6
assume 65% efficiency
Fired Heater $635 (duty, kW)0.8
assume 80% thermal efficiency
assume can be designed to use any organic compound as a fuel
Vessels $[1.67(0.959 + 0.041P - 8.3´10-6P2)]´10z
z = (3.17 + 0.2D + 0.5 log10L + 0.21 log10L2)
D = diameter, m 0.3 m < D < 4.0 m
L = height, m 3 < L/D < 20
P = absolute pressure, bar
Reactor assume to be $1 million
Equipment Cost Factors
Pressure Factors
Pressure < 10 atm, 0.0 does not apply to turbines, compressors, vessels,
(absolute) 10 - 20 atm, 0.6 packing, trays, or catalyst, since their cost
20 - 40 atm, 3.0 equations include pressure effects
40 - 50 atm, 5.0
50 - 100 atm, 10
Material Factors
Carbon Steel 0.0
Stainless Steel 4.0
Total Installed Cost = Purchased Cost (4 + material factor + pressure factor)
Heat Exchangers
For heat exchangers that do not have to be designed in detail, use the following approximations for heat transfer coefficients to allow you to determine the heat transfer area and heat exchanger cost.
| situation | h (W/m2?C) |
| condensing steam | |
| condensing organic | |
| boiling water | |
| boiling organic | |
| flowing liquid | |
| flowing gas |
Other Information
You should assume that a year equals 8000 hours. This is about 330 days, which allows for periodic shutdown and maintenance.
Unless specifically stated in class, the information in this document is valid for this project only. Any information in the sophomore projects not specifically stated in this document is not valid for this project.
Deliverables
Each group must deliver a report written using a word processor. Three identical copies should be submitted, one for each instructor. The written project reports are due by 11:00 a.m. Thursday, December 2, 1999. Late projects will receive a minimum of a one letter grade deduction.
The report should be clear and concise. For the correct formatting information, refer to the document entitled Written Design Reports. Any report not containing a labeled PFD and a stream table, each in the appropriate format, will be considered unacceptable. PFDs from CHEMCAD are generally unsuitable unless you modify them significantly. When presenting results for different cases, graphs are superior to tables. For the optimal case, the report appendix should contain details of calculations that are easy to follow. There should be separate appendices for each ?mini-design.? These may be hand written if done neatly. Calculations that cannot be easily followed will lose credit.
Since this project involves ?mini-designs,? it is suggested that the report be organized as follows. There should be a general abstract and introduction. Then, there should be a results section followed by a discussion section for each ?mini-design.? General conclusion and recommendation sections should follow. At a minimum, there should be an appendix for each of the ?mini-designs.? With this organization, there is no need for a separate section of the report for each class, as suggested in the document entitled Written Design Reports.
Each group will give an oral report in which the results of this project will be presented in a concise manner. The oral report should be between 15-20 minutes, and each group member must speak. Each group member should speak only once. A 5-10 minute question-and-answer session will follow, and all members must participate. Refer to the document entitled Oral Reports for instructions. The oral presentations will be December 2, 1999, from 11:00 a.m. to 3:00 p.m., and possibly December 3, 1999, from 11:00 to 1:00 pm. Attendance is required of all students during their classmates? presentations (this means in the room, not in the hall or the computer room). Failure to attend any of the above required sessions will result in a decrease of one-letter grade (per occurrence) from your project grade in ChE 110, ChE 111, and ChE 142.
Groups
You may do this project in a group of three or four. You should select your own group members. Since there are 31 students doing the project, there will be eight groups. Seven groups will have four members and one group will have three members.
Revisions
As with any open-ended problem (i.e., a problem with no single correct answer), the problem statement above is deliberately vague. The possibility exists that, as you work on this problem, your questions will require revisions and/or clarifications of the problem statement. You should be aware that these revisions/clarifications may be forthcoming.
Date: 2016-06-13; view: 997