
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Liquid water (left) and ice (right) structures
Water
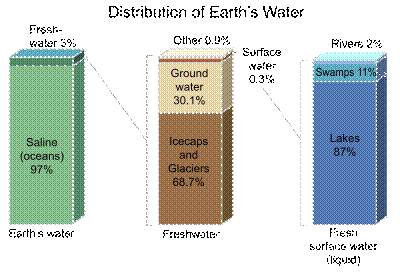
The Ancient Greek philosopher Empedocles held that water is one of the four classical elements along with fire, earth and air, and was regarded as the ylem, or basic substance of the universe. 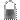
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds.
Water appears in nature in all three common states of matter (solid, liquid, and gas) and may take many different forms on Earth: water vapor and clouds in the sky; seawater in the oceans; icebergs in the polar oceans; glaciers and rivers in the mountains; and the liquid in aquifers in the ground.
Water covers 71% of the Earth's surface, and is vital for all known forms of life.
Only 3% of the Earth's water is freshwater, and 98.8% of that water is in ice and groundwater. Less than 0.3% of all freshwater is in rivers, lakes, and the atmosphere, and an even smaller amount of the Earth's freshwater (0.003%) is contained within biological bodies and manufactured products.
Water on Earth moves continually through the hydrological cycle of evaporation and transpiration, runoff, usually reaching the sea. Evaporation and transpiration contribute to the precipitation over land.
The major chemical and physical properties of water are:
· Water is, but it often coexists on Earth with its solid state, ice, and gaseous state (water vapor or steam). Water also exists in a liquid crystal state near hydrophilic surfaces.
· Water is a liquid at temperatures above 0°C at sea level. The liquid water at a little volumes is tasteless and odorless. The huge volumes of water (seas, lakes, etc.) have a very slight blue hue. Water vapor is essentially invisible as a gas.
· Water is transparent in the visible electromagnetic spectrum. Thus aquatic plants can live in water because sunlight can reach them. Infrared light is strongly absorbed by the hydrogen-oxygen or OH bonds.
· Since the water molecule is not linear and the oxygen atom has a higher electronegativity than hydrogen atoms, it carries a slight negative charge, whereas the hydrogen atoms are slightly positive. As a result, water is a polar molecule with an electrical dipole moment.
· In a liquid state one water molecule can form four intermolecular hydrogen bonds with other water molecules. These factors lead to strong attractive forces between molecules of water, giving rise to water's high surface tension and capillary forces.
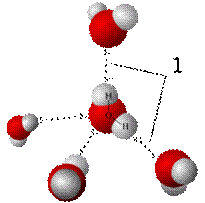
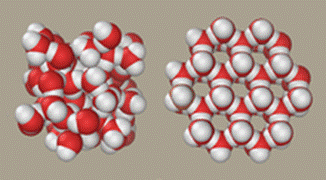
Liquid water (left) and ice (right) structures
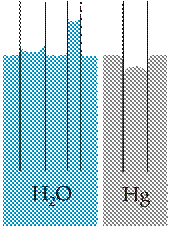
· The capillary action refers to the tendency of water to move up a narrow tube against the force of gravity. This property is relied upon by all vascular plants, such as trees.
Date: 2016-04-22; view: 1669
| <== previous page | | | next page ==> |
| Task II. ENGLISH IN USE. | | | Temperature distribution in a lake in summer and winter |