
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Call and describe the methods of measurements.
A Measurement is the outcome of an opinion formed by observers about some physical quantity.
METHODS OF MEASUREMENT: there are two main methods of measurements:
1. The direct comparison method of measurement is not always accurate. In above example of measuring the length, there is limited accuracy with which our eye can read the readings, which can be about 0.01 inch. Here the error does not occur because of the error in the standards, but because of the human limitations in noting the readings. Similarly, when we measure the mass of any body by comparing with some standard, it’s very difficult to say that both the bodies are of exactly the same mass, for some difference between the two, no matter how small, is bound to occur. Thus, in direct method of measurement there is always some difference, however small, between the actual value of the quantity and the measured value of the quantity.
2. There are number of quantities that cannot be measured directly by using some instrument. In such cases indirect methodsof measurements are used.
In the indirect method of measurements some transducing devise, called transducer, is used, which is coupled to a chain of the connecting apparatus that forms the part of the measuring system. In this system the quantity which is to be measured (input) is converted into some other measurable quantity (output) by the transducer. The transducer used is such that the input and the output are proportional to each other. Such type of conversion is often necessary to make the desired information intelligible.
Also there are other methods such as:
3. Comparative Method: It’s compared with other known value. Ex: Comparators.
4. . Deflection Method: The value to be measured is directly indicated by a deflection of pointer. Ex: Pressure Measurement.
5. Fundamental Method: Measuring a quantity directly in related with the definition of that quantity.
6. Transposition Method: Quantity to be measured is first balanced by a known value and then balanced by any other new known value.
Ex: Determination of mass by balancing methods.
7. Complementary Method: The value of quantity to be measured is combined with known value of the same quantity.
Ex: Volume determination by liquid displacement.
4. Call the measuring instruments. Describe metrological characteristics of measuring instruments. Compare errors of measuring instruments.
Measuring instruments in chemistry laboratory: burettes, pipettes, thermometers, pH meter, balances, etc. By metrological characteristics of a measuring instrument, we mean the characteristics that make it possible to judge the suitability of the instrument for performing measurements in a known range with known accuracy, to obtain a value of the measure, and to estimate its inaccuracy.
Let’s consider the mechanism of weighing balance. To weigh a chemical, first place a clean receiving vessel on the balance pan. The mass of the empty vessel is called the tare. On most balances, you can press a button to reset the tare to 0. Add the chemical to the vessel and read its mass. If there is no automatic tare operation, subtract the tare mass from that of the filled vessel. To protect the balance from corrosion, chemicals should never be placed directly on the weighing pan. Also, be careful never to spill chemicals into the mechanism below the balance pan.
How a Mechanical Balance Works? The classic mechanical balance has two pans suspended on opposite ends of an equal-arm lever balanced at its center on a knife edge. An unknown mass is placed on one pan and standard masses are placed on the other pan. When the balance is restored to its level position, the mass of the standards is equal to the mass of the unknown.
How an Electronic Balance Works? An object placed on the electronic balance pushes the pan down with a force m x g, where m is the mass of the object and g is the acceleration of gravity.
Errors: There are three types of errors in the measuring instruments: assembly errors, environmental errors, and random errors.
1) Assembly Errors. The assembly errors are the errors in the measuring instrument due to improper manufacturing of the instruments. If there are assembly errors in the instruments, the instrument will just not give the correct reading and the user can hardly do anything about it.
2) Environmental Errors. The measuring instruments are assembled and calibrated in certain environmental conditions and are designed to be used in within certain restricted conditions, but when they are used in different conditions, there are errors in measurement, which are considered to be the environmental errors. Most of the instruments are designed to be used within certain limits of temperature, pressure, humidity, altitude etc and when the limits are extended there are errors in the measuring instruments.
3) Random Errors. Apart from the assembly and environmental errors there can be many other errors which may be very difficult to trace and predict, these are called as random errors.
5. What is concentration? Give a definition for types of concentration?
A solution is a homogeneous mixture of two or more substances. A minor species in a solution is called solute, and the major species is the solvent. Concentration states how much solute is
contained in a given volume or mass of solution or solvent.
A mole (mol) is Avogadro’s number of particles (atoms, molecules, ions, or anything else). Molarity (M) is the number of moles of a substance per liter of solution. A liter (L) is the volume of a cube that is 10 cm on each edge. Because 10 cm=0.1 m, 1 L =  =
=  . Chemical concentrations, denoted with square brackets, are usually expressed in moles per liter (M). Thus “[
. Chemical concentrations, denoted with square brackets, are usually expressed in moles per liter (M). Thus “[  ]” means “the concentration of
]” means “the concentration of  ”
”
The atomic mass of an element is the number of grams containing Avogadro’s number of atoms.1The molecular mass of a compound is the sum of atomic masses of the atoms in the
molecule. It is the number of grams containing Avogadro’s number of molecules. An electrolyte is a substance that dissociates into ions in solution. In general, electrolytes
are more dissociated in water than in other solvents. We refer to a compound that is mostly dissociated into ions as a strong electrolyte. One that is partially dissociated is called a weak
electrolyte. Magnesium chloride is a strong electrolyte. In 0.44 M MgCl2solution, 70% of the magnesium is free Mg2?and 30% is MgCl?. The concentration of MgCl2molecules is close to 0. Sometimes the molarity of a strong electrolyte is called the formal concentration (F), to emphasize that the substance is really converted into other species in solution. When we say that the “concentration” of MgCl2is 0.054 M in seawater, we are really speaking of its formal concentration (0.054 F). The “molecular mass” of a strong electrolyte is called the formula mass (FM), because it is the sum of atomic masses of atoms in the formula, even though there are very few molecules with that formula.
Molality (m) is concentration expressed as moles of substance per kilogram of solvent (not total solution). Molality is independent of temperature. Molarity changes with temperature because the volume of a solution usually increases when it is heated. Percent Composition The percentage of a component in a mixture or solution is usually expressed as a weight percent (wt%):

A common form of ethanol (CH3CH2OH) is 95 wt%; this expression means 95 g of ethanol per 100 g of total solution. The remainder is water. Volume percent (vol%) is defined as

Although units of mass or volume should always be expressed to avoid ambiguity, mass is usually implied when units are absent.
6. How to prepare a solution with a desired molarity?
To prepare a solution with a desired molarity from a pure solid or liquid, we weigh out the correct mass of reagent and dissolve it in a volumetric flask. Copper(II) sulfate pentahydrate, CuSO4*5H2O, has 5 moles of for each mole of CuSO4in the solid crystal. The formula mass of CuSO4*5H2O is 249.68 g/mol. (Copper(II) sulfate without water in the crystal has the formula CuSO4and is said to be anhydrous.) How many grams of CuSO4*5H2O should be dissolved in a volume of 500.0 mL to make 8.00 mM 
Solution
An 8.00 mM solution contains 8.00*  We need
We need
8.00* 
 * 0.5000 L=4.00*
* 0.5000 L=4.00*  mol CuSO4*5H2O
mol CuSO4*5H2O
The mass of reagent is (4.00*  mol)*(249.68 g/mol)=0.999 g.
mol)*(249.68 g/mol)=0.999 g.
Using a volumetric flask: The procedure is to place 0.999 g of solid CuSO4*5H2O into a 500-mL volumetric flask, add about 400 mL of distilled water, and swirl to dissolve the reagent. Then dilute with distilled water up to the 500-mL mark and invert the flask several times to ensure complete mixing.
ilute solutions can be prepared from concentrated solutions. A volume of the concentrated solution is transferred to a fresh vessel and diluted to the desired final volume. The number of
moles of reagent in V liters containing M moles per liter is the product M*V=mol/L*L, so we equate the number of moles in the concentrated (conc) and dilute (dil) solutions:
Dilution formula:

7. Explain the differences between electronic and mechanic balances . What is the buoyancy?
An electronic balance uses electromagnetic force compensation to balance the load on the pan. Readability is the smallest increment of mass that can be indicated. A microbalance weighs milligram quantities with a readability of 1  g.
g.
To weigh a chemical, first place a clean receiving vessel on the balance pan. The mass of the empty vessel is called the tare. On most balances, you can press a button to reset the tare to 0. Add the chemical to the vessel and read its mass. If there is no automatic tare operation, subtract the tare mass from that of the filled vessel. To protect the balance from corrosion, chemicals should never be placed directly on the weighing pan. Also, be careful never to spill chemicals into the mechanism below the balance pan. An alternate procedure, called weighing by difference, is necessary for hygroscopic reagents, which rapidly absorb moisture from the air. First weigh a capped bottle containing dry reagent. Then quickly pour some reagent from the weighing bottle into a receiver. Cap the weighing bottle and weigh it again. The difference is the mass of reagent delivered from the weighing bottle. With an electronic balance, set the initial mass of the weighing bottle to 0 with the tare button. Then deliver reagent from the bottle and reweigh the bottle. The negative reading on the balance is the mass of reagent delivered from the bottle. The classic mechanical balance has two pans suspended on opposite ends of an equal-arm lever balanced at its center on a knife edge. An unknown mass is placed on one pan and standard masses are placed on the other pan. When the balance is restored to its level position, the mass of the standards is equal to the mass of the unknown. The mass of the pan at the left is balanced by a counterweight at the right. An object to be weighed is placed on the pan. We then rotate mechanical knobs to detach calibrated internal weights until the balance beam is restored as close as possible to its original position. The remaining small deflection is read on the optical scale and this reading is added to that of the removed weights.
A mechanical balance should be in its arrested position when you load or unload the pan and in the half-arrested position when you are dialing weights. You can float in water because your weight when swimming is nearly zero. Buoyancy is the upward force exerted on an object in a liquid or gaseous fluid.11An object weighed in air appears lighter than its actual mass by an amount equal to the mass of air that it displaces. True mass is the mass measured in vacuum. A standard mass in a balance is also
affected by buoyancy, so it weighs less in air than in vacuum. A buoyancy error occurs whenever the density of the object being weighed is not equal to the density of the standard mass.
If mass 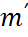 is read on a balance, the true mass m of the object weighed in vacuum is
is read on a balance, the true mass m of the object weighed in vacuum is
Buoyancy equation:

where da is the density of air, dw is the density of the calibration weights and d is the density of the object being weighed.
8. What is the error? Give a definition of types of error.






9) Explain the differences between precision and accuracy. Precision describes the reproducibility of a result. If you measure a quantity several times and the values agree closely with one another, your measurement is precise. If the values vary widely, your measurement is not precise. Accuracy describes how close a measured value is to the “true” value. If a known standard is available, accuracy is how close your value is to the known value. The U.S. National Institute of Standards and Technology and national standards laboratories in other countries sell certified reference materials (called Standard Reference Materials in the U.S.), such as clinical and environmental standards and engineering materials that you can use to test the accuracy of your analytical procedures. The quantity of analyte in a reference material is certified—with painstaking care—to lie in a stated range. A measurement might be reproducible, but wrong. If you made a mistake preparing a solution for a titration, you might do a series of reproducible titrations but report an incorrect result because the concentration of the titrating solution was not what you intended. In this case, precision is good but accuracy is poor. Conversely, it is possible to make poorly reproducible measurements clustered around the correct value. For this case, precision is poor but accuracy is good. An ideal procedure is both precise and accurate. Accuracy is defined as nearness to the “true” value. True is in quotes because somebody must measure the “true” value, and there is error associated with every measurement. The “true” value is best obtained by an experienced person using a well-tested procedure. It is desirable to test the result by using different procedures, because systematic error could lead to poor agreement between methods. Good agreement among several methods affords us confidence, but never proof, that results are accurate.
10) What is the uncertainty? How to calculate it?
Absolute uncertainty expresses the margin of uncertainty associated with a measurement. If the estimated uncertainty in reading a calibrated buret is ± 0.02 mL, we say that ± 0.02 mL is the absolute uncertainty associated with the reading. Relative uncertainty compares the size of the absolute uncertainty with the size of its associated measurement. The relative uncertainty of a buret reading of 12.35 ± 0.02 mL is a dimensionless quotient:

The percent relative uncertainty is simply

Uncertainty might be based on how well we can read an instrument or on our experience with a particular method. For most experiments, we need to perform arithmetic operations on several numbers, each of which has a random error.
Addition and Subtraction Suppose you wish to perform the following arithmetic, in which the experimental uncertainties, designated e1, e2, and e3, are given in parentheses.

The arithmetic answer is 3.06. But what is the uncertainty associated with this result? For addition and subtraction, the uncertainty in the answer is obtained from the absolute uncertainties of the individual terms as follows:


The absolute uncertainty e4 is ±0.04, and we express the answer as 3.06 ± 0.04.

Multiplication and Division For multiplication and division, first convert all uncertainties into percent relative uncertainties. Then calculate the error of the product or quotient as follows:
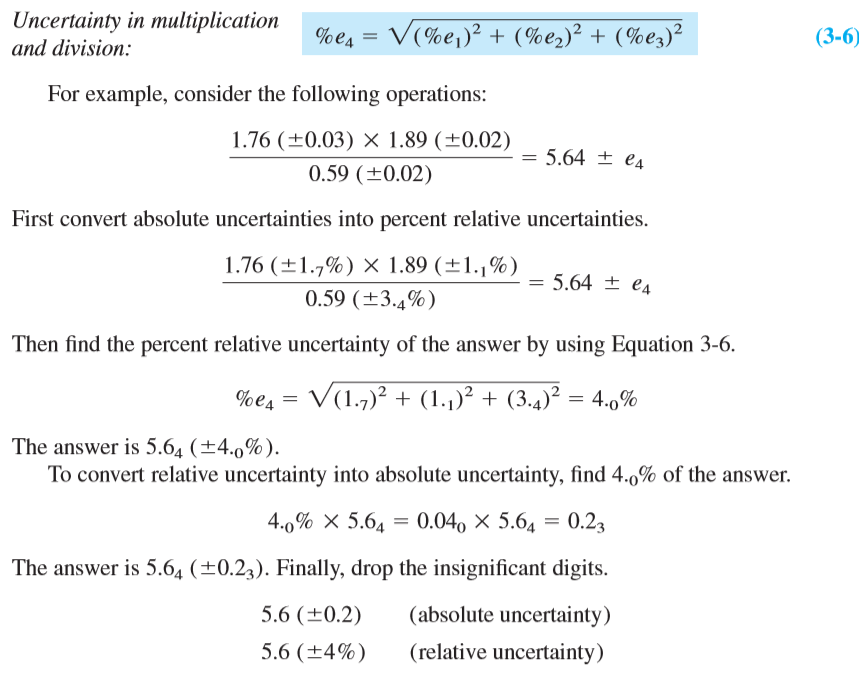
Summary of rules for propagation of uncertainty


Date: 2016-01-14; view: 2661
| <== previous page | | | next page ==> |
| List the main SI units of measurements. | | | Explain the meaning of Gaussian Distribution. Gaussian curve. |