
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
II. Correct the following statements.
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of _____ light dispersed according to its wavelength, e.g., by a prism. Analysis of white light by dispersing it with a prism is an example of spectroscopy. Later the concept was expanded greatly to comprise any interaction with radiative energy as a function of its wavelength or frequency. Spectroscopic data is often represented by a spectrum, a plot of the response of interest as a function of wavelength or frequency.
Spectrometry and spectrography are terms used to refer to the measurement of ______ intensity as a function of wavelength and are often used to describe experimental spectroscopic methods. Spectral measurement devices are referred to as spectrometers, spectrophotometers, spectrographs or spectral analyzers.
One of the central concepts in spectroscopy is a resonance and its corresponding resonant ______. Resonances were first characterized in mechanical systems such as pendulums. Mechanical systems that vibrate or oscillate will experience large amplitude oscillations when they are driven at their resonant frequency. A plot of amplitude vs. excitation frequency will have a peak centered at the resonance frequency. This plot is one type of spectrum, with the peak often referred to as a spectral line, and most spectral lines have a similar appearance.
In quantum mechanical systems, the analogous resonance is a coupling of two quantum mechanical stationary states of one system, such as an atom, via an oscillatory source of energy such as a photon. The coupling of the two states is strongest when the energy of the source matches the energy difference between the two states. The energy (  ) of a ______ is related to its frequency (
) of a ______ is related to its frequency (  ) by
) by  where
where 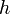 is Planck's constant, and so a spectrum of the system response vs. photon frequency will peak at the resonant frequency or energy. Particles such as electrons and neutrons have a comparable relationship, the de Broglie relations, between their kinetic energy and their wavelength and frequency and therefore can also excite resonant interactions.
is Planck's constant, and so a spectrum of the system response vs. photon frequency will peak at the resonant frequency or energy. Particles such as electrons and neutrons have a comparable relationship, the de Broglie relations, between their kinetic energy and their wavelength and frequency and therefore can also excite resonant interactions.
Spectra of atoms and molecules often consist of a series of _____ lines, each one representing a resonance between two different quantum states. The explanation of these series, and the spectral patterns associated with them, were one of the experimental enigmas that drove the development and acceptance of quantum mechanics. The hydrogen spectral series in particular was first successfully explained by the Rutherford-Bohr quantum model of the hydrogen atom. In some cases spectral lines are well separated and distinguishable, but spectral lines can also overlap and appear to be a single transition if the density of energy states is high enough.
Spectroscopy is a sufficiently broad field that many sub-disciplines exist, each with numerous implementations of specific spectroscopic techniques. The various implementations and techniques can be classified by the type of radiative _____ involved in the interaction, by the nature of the interaction, such as absorption or emission, or by the type of material.
In many applications, the spectrum is determined by measuring changes in the _______ or frequency of this energy. For example, techniques that employ electromagnetic radiation are typically classified by the wavelength region of the spectrum and include microwave, terahertz, infrared, near infrared, visible and ultraviolet, x-ray and gamma spectroscopy.
Spectroscopic studies are designed so that the radiant energy interacts with specific types of matter. Atomic spectroscopy, the first application of spectroscopy developed, involves visible and ultraviolet light. Atoms of different elements have distinct spectra and therefore atomic spectroscopy allows for the _____ and quantitation of a sample's elemental composition. Robert Bunsen, developer of the Bunsen burner, and Gustav Kirchhoff discovered new elements by observing their emission spectra. Atomic absorption lines are observed in the solar spectrum and referred to as Fraunhofer lines after their discoverer.
The combination of atoms into molecules leads to the creation of _____ types of energetic states and therefore unique spectra of the transitions between these states. Molecular spectra can be obtained due to electron spin states (electron paramagnetic resonance), molecular rotations, molecular vibration and electronic states. Studies in molecular spectroscopy led to the development of the first maser and contributed to the subsequent development of the laser.
Daily observations of color can be related to spectroscopy. Neon lighting is a direct application of atomic spectroscopy. Neon and other ____ gases have characteristic emission colors, and neon lamps use electricity to excite these emissions. Inks, dyes and paints include chemical compounds selected for their spectral characteristics in order to generate specific colors and hues. A commonly encountered molecular spectrum is that of nitrogen dioxide. Gaseous nitrogen dioxide has a characteristic red absorption feature, and this gives air polluted with nitrogen dioxide a reddish brown color. Rayleigh scattering is a spectroscopic scattering phenomenon that accounts for the color of the sky.
Spectroscopic studies were central to the development of quantum mechanics and included Max Planck's explanation of blackbody radiation, Albert Einstein's explanation of the photoelectric effect and Niels Bohr's explanation of atomic structure and spectra. Spectroscopy is used in astronomy and _____ sensing. Most research telescopes have spectrographs. The measured spectra are used to determine the chemical composition and physical properties of astronomical objects (such as their temperature and velocity).
II. Correct the following statements.
1. Radiation intensity is not connected to wavelength.
2. Electrons canít excite resonant interactions.
3. Spectral lines are always easy to distinguish.
4. The implementations and techniques of spectroscopy are classified only by the type of material.
5. The visible color of objects is not connected to spectral characteristics of substance they consist of.
Date: 2015-12-24; view: 1023
| <== previous page | | | next page ==> |
| Create Master Password dialog | | | Part 2. Individual long turn |