
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Superconductivity
| I | INTRODUCTION |
Superconductivity, phenomenon displayed by certain conductors that demonstrate no resistance to the flow of an electric current. Superconductors also exhibit strong diamagnetism; that is, they are repelled by magnetic fields. Superconductivity is manifested only below a certain critical temperature Tc and a critical magnetic field Hc, which vary with the material used. Before 1986, the highest Tc was 23.2 K (-249.8° C/-417.6° F) in niobium-germanium compounds. Temperatures this low were achieved by use of liquid helium, an expensive, inefficient coolant. Ultralow-temperature operation places a severe constraint on the overall efficiency of a superconducting machine. Thus, large-scale operation of such machines was not considered practical. But in 1986 discoveries at several universities and research centers began to radically alter this situation. Ceramic metal-oxide compounds containing rare earth elements were found to be superconductive at temperatures high enough to permit using liquid nitrogen as a coolant. Because liquid nitrogen, at 77K (-196° C/-321° F), cools 20 times more effectively than liquid helium and is 10 times less expensive, a host of potential applications suddenly began to hold the promise of economic feasibility. In 1987 the composition of one of these superconducting compounds, with Tc of 94K (-179° C/-290° F), was revealed to be YBa2Cu307 (yttrium-barium-copper-oxide). It has since been shown that rare-earth elements, such as yttrium, are not an essential constituent, for in 1988 a thallium-barium-calcium copper oxide was discovered with a Tc of 125K (-148° C/-234° F).
| II | HISTORY |
Superconductivity was first discovered in 1911 by the Dutch physicist Heike Kamerlingh Onnes, who observed no electrical resistance in mercury below 4.2 K (-268.8° C/-451.8° F). The phenomenon was better understood only after strong diamagnetism was detected in a superconductor by Karl W. Meissner and R. Ochsenfeld of Germany in 1933. The basic physics of superconductivity, however, was not realized until 1957, when the American physicists John Bardeen, Leon N. Cooper, and John R. Schrieffer advanced the now celebrated BCS theory, for which the three were awarded the 1972 Nobel Prize in physics. The theory describes superconductivity as a quantum phenomenon (see Quantum Theory), in which the conduction electrons move in pairs and thus show no electrical resistance. In 1962 the British physicist Brian D. Josephson examined the quantum nature of superconductivity and proposed the existence of oscillations in the electric current flowing through two superconductors separated by a thin insulating layer in a magnetic or electric field. The effect, known as the Josephson effect, subsequently was confirmed by experiments.
| III | APPLICATIONS |
Because of their lack of resistance, superconductors have been used to make electromagnets that generate large magnetic fields with no energy loss. Superconducting magnets have been used in diagnostic medical equipment, studies of materials, and in the construction of powerful particle accelerators. Using the quantum effects of superconductivity, devices have been developed that measure electric current, voltage, and magnetic field with unprecedented sensitivity.
The discovery of better superconducting compounds is a significant step toward a far wider spectrum of applications, including faster computers with larger storage capacities, nuclear fusion reactors in which ionized gas is confined by magnetic fields, magnetic levitation (lifting or suspension) of high-speed (“Maglev”) trains, and perhaps most important of all, more efficient generation and transmission of electric power. The 1987 Nobel Prize in physics went to West German physicist J. Georg Bednorz and Swiss physicist K. Alex Müller for their discovery of materials that are superconductive at temperatures higher than had been thought possible. See Electricity; Magnetism.
Metals
| I | INTRODUCTION |
Metals, group of chemical elements that exhibit all or most of the following physical qualities: they are solid at ordinary temperatures; opaque, except in extremely thin films; good electrical and thermal conductors (see Conductor, Electrical); lustrous when polished; and have a crystalline structure when in the solid state. Metals and nonmetals are separated in the periodic table by a diagonal line of elements. Elements to the left of this diagonal are metals, and elements to the right are nonmetals. Elements that make up this diagonal—boron, silicon, germanium, arsenic, antimony, tellurium, polonium, and astatine—have both metallic and nonmetallic properties. The common metallic elements include the following: aluminum, barium, beryllium, bismuth, cadmium, calcium, cerium, chromium, cobalt, copper, gold, iridium, iron, lead, lithium, magnesium, manganese, mercury, molybdenum, nickel, osmium, palladium, platinum, potassium, radium, rhodium, silver, sodium, tantalum, thallium, thorium, tin, titanium, tungsten, uranium, vanadium, and zinc. Metallic elements can combine with one another and with certain other elements, either as compounds, as solutions, or as intimate mixtures. A substance composed of two or more metals, or a substance composed of a metal and certain nonmetals such as carbon are called alloys. Alloys of mercury with other metallic elements are known as amalgams.
Within the general limits of the definition of a metal, the properties of metals vary widely. Most metals are grayish in color, but bismuth is pinkish, copper is red, and gold is yellow. Some metals display more than one color, a phenomenon called pleochroism. The melting points of metals range from about -39° C (about -38° F) for mercury to 3410° C (6170° F) for tungsten. Osmium and iridium (specific gravity 22.6) are the most dense metals, and lithium (specific gravity 0.53) is the least dense. The majority of metals crystallize in the cubic system, but some crystallize in the hexagonal and tetragonal systems (see Crystal). Bismuth has the lowest electrical conductivity of the metallic elements, and silver the highest at ordinary temperatures. (For conductivity at low temperatures, see Cryogenics; Superconductivity.) The conductivity of most metals can be lowered by alloying. All metals expand when heated and contract when cooled, but certain alloys, such as platinum and iridium alloys, have extremely low coefficients of expansion.
| II | PHYSICAL PROPERTIES |
Metals are generally very strong and resistant to different types of stresses. Though there is considerable variation from one metal to the next, in general metals are marked by such properties as hardness, the resistance to surface deformation or abrasion; tensile strength, the resistance to breakage; elasticity, the ability to return to the original shape after deformation; malleability, the ability to be shaped by hammering; fatigue resistance, the ability to resist repeated stresses; and ductility, the ability to undergo deformation without breaking. See Materials Science and Technology.
| III | CHEMICAL PROPERTIES |
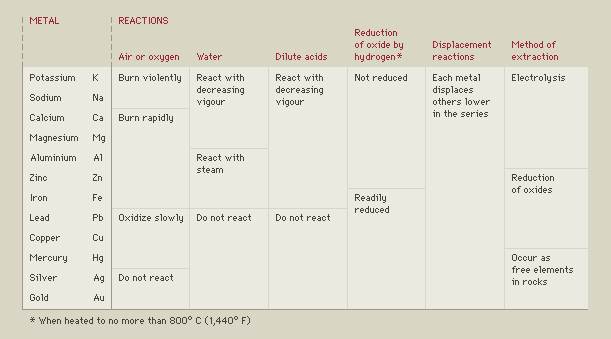
Reactivity Series
Chemists can list metals according to how quickly they undergo chemical reactions, such as burning or dissolving in acids. The result is called a reactivity series. A metal at the top of the series generally reacts more vigorously than those that are below it in the series, and the more reactive metal can take their place (or displace them) in various compounds or in solution. In some reactions, however, such as reduction reactions, the order of reactivity is reversed.
Metals typically have positive valences in most of their compounds, which means they tend to donate electrons to the atoms to which they bond. Also, metals tend to form basic oxides. Typical nonmetallic elements, such as nitrogen, sulfur, and chlorine, have negative valences in most of their compounds—meaning they tend to accept electrons—and form acidic oxides (see Acids and Bases; Chemical Reaction).
Metals typically have low ionization potentials. This means that metals react easily by loss of electrons to form positive ions, or cations. Thus, metals can form salts (chlorides, sulfides, and carbonates, for example) by serving as reducing agents (electron donors).
| IV | ELECTRON STRUCTURE |
In early attempts to explain the electronic configurations of the metals, scientists cited the characteristics of high thermal and electrical conductivity in support of a theory that metals consist of ionized atoms in which the free electrons form a homogeneous sea of negative charge. The electrostatic attraction between the positive metal ions and the free-moving and homogeneous sea of electrons was thought to be responsible for the bonds between the metal atoms. Free movement of the electrons was then held to be responsible for the high thermal and electrical conductivities. The principal objection to this theory was that the metals should then have higher specific heats than they do.
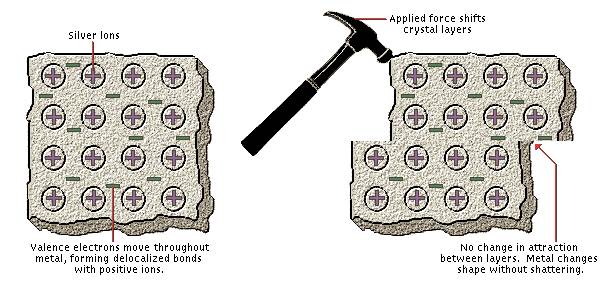
Metallic Bonding
Silver, a typical metal, consists of a regular array of silver atoms that have each lost an electron to form a silver ion. The negative electrons distribute themselves throughout the entire piece of metal and form nondirectional bonds between the positive silver ions. This arrangement, known as metallic bonding, accounts for the characteristic properties of metals: they are good electrical conductors because the electrons are free to move from one place to another, and they are malleable (as shown here) because the positive ions are held together by nondirectional forces.
In 1928 the German physicist Arnold Sommerfeld proposed that the electrons in metals exist in a quantized arrangement in which low energy levels available to the electrons are almost fully occupied (see Atom; Quantum Theory). In the same year the Swiss-American physicist Felix Bloch and later the French physicist Louis Brillouin used this idea of quantization in the currently accepted “band” theory of bonding in metallic solids.
According to the band theory, any given metal atom has only a limited number of valence electrons with which to bond to all of its nearest neighbors. Extensive sharing of electrons among individual atoms is therefore required. This sharing of electrons is accomplished through overlap of equivalent-energy atomic orbitals on the metal atoms that are immediately adjacent to one another. This overlap is delocalized throughout the entire metal sample to form extensive orbitals that span the entire solid rather than being part of individual atoms. Each of these orbitals lies at different energies because the atomic orbitals from which they were constructed were at different energies to begin with. The orbitals, equal in number to the number of individual atomic orbitals that have been combined, each hold two electrons, and are filled in order from lowest to highest energy until the number of available electrons has been used up. Groups of electrons are then said to reside in bands, which are collections of orbitals. Each band has a range of energy values that the electrons must possess to be part of that band; in some metals, there are energy gaps between bands, meaning that there are certain energies that the electrons cannot possess. The highest energy band in a metal is not filled with electrons because metals characteristically possess too few electrons to fill it. The high thermal electrical conductivities of metals is then explained by the notion that electrons may be promoted by absorption of thermal energy into these unfilled energy levels of the band.
Date: 2015-01-02; view: 1508
| <== previous page | | | next page ==> |
| Friction | | | Temperature |