
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Equilibrium Constant of Concentration
LAB # 3
law of mass action
1.the statement that the rate of a chemical reaction is proportional tothe concentrations of the reacting substances.
Using Le Chatelier's Principle
A statement of Le Chatelier's Principle
 
| |
| Note:In case you wonder, the reason for choosing this equation rather than having just A + B on the left-hand side is because further down this page I need an equation which has different numbers of molecules on each side. I am going to use that same equation throughout this page. | |
What would happen if you changed the conditions by increasing the concentration of A?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the concentration of A decreases again - by reacting it with B and turning it into C + D. The position of equilibrium moves to the right.
 This is a useful way of converting the maximum possible amount of B into C and D. You might use it if, for example, B was a relatively expensive material whereas A was cheap and plentiful.
What would happen if you changed the conditions by decreasing the concentration of A?
According to Le Chatelier, the position of equilibrium will move so that the concentration of A increases again. That means that more C and D will react to replace the A that has been removed. The position of equilibrium moves to the left.
This is a useful way of converting the maximum possible amount of B into C and D. You might use it if, for example, B was a relatively expensive material whereas A was cheap and plentiful.
What would happen if you changed the conditions by decreasing the concentration of A?
According to Le Chatelier, the position of equilibrium will move so that the concentration of A increases again. That means that more C and D will react to replace the A that has been removed. The position of equilibrium moves to the left.
 This is esssentially what happens if you remove one of the products of the reaction as soon as it is formed. If, for example, you removed C as soon as it was formed, the position of equilibrium would move to the right to replace it. If you kept on removing it, the equilibrium position would keep on moving rightwards - turning this into a one-way reaction.
Important
This isn't in any way an explanation of why the position of equilibrium moves in the ways described. All Le Chatelier's Principle gives you is a quick way of working out what happens.
This is esssentially what happens if you remove one of the products of the reaction as soon as it is formed. If, for example, you removed C as soon as it was formed, the position of equilibrium would move to the right to replace it. If you kept on removing it, the equilibrium position would keep on moving rightwards - turning this into a one-way reaction.
Important
This isn't in any way an explanation of why the position of equilibrium moves in the ways described. All Le Chatelier's Principle gives you is a quick way of working out what happens.
| |
| Note:If you know about equilibrium constants, you will find a more detailed explanation of the effect of a change of concentration by following this link. If you don't know anything about equilibrium constants, you should ignore this link. If you choose to follow it, return to this page via the BACK button on your browser or via the equilibrium menu. | |
Using Le Chatelier's Principle with a change of pressure
This only applies to reactions involving gases:
  What would happen if you changed the conditions by increasing the pressure?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again.
Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
In this case, there are 3 molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce.
Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules.
What would happen if you changed the conditions by increasing the pressure?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again.
Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
In this case, there are 3 molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce.
Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules.
 What would happen if you changed the conditions by decreasing the pressure?
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.
What would happen if you changed the conditions by decreasing the pressure?
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.
 What happens if there are the same number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect whatsoever on the position of the equilibrium. Because you have the same numbers of molecules on both sides, the equilibrium can't move in any way that will reduce the pressure again.
Important
Again, this isn't an explanation of why the position of equilibrium moves in the ways described. You will find a rather mathematical treatment of the explanation by following the link below.
What happens if there are the same number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect whatsoever on the position of the equilibrium. Because you have the same numbers of molecules on both sides, the equilibrium can't move in any way that will reduce the pressure again.
Important
Again, this isn't an explanation of why the position of equilibrium moves in the ways described. You will find a rather mathematical treatment of the explanation by following the link below.
| |
| Note:You will find a detailed explanation by following this link. If you don't know anything about equilibrium constants (particularly Kp), you should ignore this link. The same thing applies if you don't like things to be too mathematical! If you are a UK A' level student, you won't need this explanation. If you choose to follow the link, return to this page via the BACK button on your browser or via the equilibrium menu. | |
Using Le Chatelier's Principle with a change of temperature
For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):
  This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction.
The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount.
This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction.
The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount.
 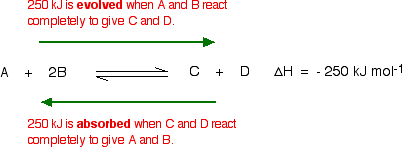 What would happen if you changed the conditions by increasing the temperature?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again.
Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again?
To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reactionabsorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.
What would happen if you changed the conditions by increasing the temperature?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again.
Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again?
To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reactionabsorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.
 If you were aiming to make as much C and D as possible, increasing the temperature on a reversible reaction where the forward reaction is exothermic isn't a good idea!
What would happen if you changed the conditions by decreasing the temperature?
The equilibrium will move in such a way that the temperature increases again.
Suppose the system is in equilibrium at 500°C and you reduce the temperature to 400°C. The reaction will tend to heat itself up again to return to the original temperature. It can do that by favouring the exothermic reaction.
The position of equilibrium will move to the right. More A and B are converted into C and D at the lower temperature.
If you were aiming to make as much C and D as possible, increasing the temperature on a reversible reaction where the forward reaction is exothermic isn't a good idea!
What would happen if you changed the conditions by decreasing the temperature?
The equilibrium will move in such a way that the temperature increases again.
Suppose the system is in equilibrium at 500°C and you reduce the temperature to 400°C. The reaction will tend to heat itself up again to return to the original temperature. It can do that by favouring the exothermic reaction.
The position of equilibrium will move to the right. More A and B are converted into C and D at the lower temperature.
 Summary
Summary
|
2.
Equilibrium Constant of Concentration
The equilibrium constant of concentration gives the ratio of concentrations of products over reactants for a reaction that is at equilibrium. This is usually used when the state of matter for the reaction is (aq). The equilibrium constant expression is written as Kc, as in the expression below:
Kc= [G]g[H]h
[A]a[B]b
· If K>1 then equilibrium favors products
· If K<1 then equilibrium favors the reactants
Here, the letters inside the brackets represent the concentration of each molecule. Notice the mathematical product of the chemical products raised to the powers of their respective coefficients is the numerator of the ratio and the mathematical product of the reactants raised to the powers of their respective coefficients is the denominator. This is the case for every equilibrium constant. Keep in mind that this expression was obtained by a homogeneous equilibrium reaction. K represents an equilibrium constant and c represents concentration (e.g., Kc). This means that every speciesshows up in the expression, as long as it is a solution or a gas.
4. Van't Hoff Rule states that when the temperature rises to 10 degrees every chemical reaction rate increases by 2-4 times.
Mathematically, the van't Hoff rule is as follows:

Lab # 4
1. solution is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The solution more or less takes on the characteristics of the solvent including its phase, and the solvent is commonly the major fraction of the mixture. The concentration of a solute in a solution is a measure of how much of that solute is dissolved in the solvent, with regard to how much solvent is present.
2. 
Molarity
Molarity tells us the number of moles of solute in exactly one liter of a solution. (Note that molarity is spelled with an "r" and is represented by a capital M.)
We need two pieces of information to calculate the molarity of a solute in a solution:
- The moles of solute present in the solution.
- The volume of solution (in liters) containing the solute.
To calculate molarity we use the equation:

Molality
Molality, m, tells us the number of moles of solute dissolved in exactly one kilogram of solvent. (Note that molality is spelled with two "l"'s and represented by a lower case m.)
We need two pieces of information to calculate the molality of a solute in a solution:
- The moles of solute present in the solution.
- The mass of solvent (in kilograms) in the solution.
To calculate molality we use the equation:

 Normal concentration
Normal concentration
3. Strong Electrolytes Strong electrolytes are substances that only exist as ions in solution. Ionic compounds are typically strong electrolytes. Strong acids, strong bases and salts are strong electrolytes. When solid NaCl is placed in water, it completely dissociates to form Na + and Cl- ions. H2O NaCl(s) → Na+ (aq) + Cl- (aq) Weak Electrolytes
A weak electrolyte only partially dissociates in solution and produces relatively few ions. Polar covalent compounds are typically weak electrolytes. Weak acids and weak bases are weak electrolytes. H2O CH3COOH(l) CH3COO- (aq) + H+ (aq) Nonelectrolytes A nonelectrolyte does not dissociate at all in solution and therefore does not produce any ions.
Nonelectrolytes are typically polar covalent substances that do dissolve in water as molecules instead of ions. Sugar (C12H22O11) is a good example of a nonelectrolyte.
4. A measure of acidity or alkalinity of water soluble substances (pH stands for 'potential of Hydrogen'). A pH value is a number from 1 to 14, with 7 as the middle (neutral) point. Values below 7 indicate acidity which increases as the number
decreases, 1 being the most acidic. Values above 7 indicate alkalinity which increases as the number increases, 14 being the most alkaline. This scale, however, is not a linear scale like a centimeter or inch scale (in which two adjacent values have the same difference). It is a logarithmic scale in which two adjacent values increase or decrease by a factor of 10. For example, a pH of 3 is ten times more acidic than a pH of 4, and 100 times more acidic than a pH of 5. Similarly, a pH of 9 is 10 times more alkaline than a pH of 8
5. , entropy is commonly associated with the amount of order, disorder, or chaos in a thermodynamic system.
Owing to these early developments, the typical example of entropy change ΔS is that associated with phase change. In solids, for example, which are typically ordered on the molecular scale, usually have smaller entropy than liquids, and liquids have smaller entropy than gases and colder gases have smaller entropy than hotter gases. Moreover, according to the third law of thermodynamics, at absolute zero temperature, crystalline structures are approximated to have perfect "order" and zero entropy. This correlation occurs because the numbers of different microscopic quantum energy states available to an ordered system are usually much smaller than the number of states available to a system that appears to be disordered.
From his famous 1896 Lectures on Gas Theory, Boltzmann diagrams the structure of a solid body, as shown above, by postulating that each molecule in the body has a "rest position". According to Boltzmann, if it approaches a neighbor molecule it is repelled by it, but if it moves farther away there is an attraction. This, of course was a revolutionary perspective in its time; many, during these years, did not believe in the existence of either atoms or molecules (see: history of the molecule).[16] According to these early views, and others such as those developed by William Thomson, if energy in the form of heat is added to a solid, so to make it into a liquid or a gas, a common depiction is that the ordering of the atoms and molecules becomes more random and chaotic with an increase in temperature:
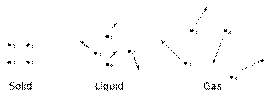
Thus, according to Boltzmann, owing to increases in thermal motion, whenever heat is added to a working substance, the rest position of molecules will be pushed apart, the body will expand, and this will create more molar-disordered distributions and arrangements of molecules. These disordered arrangements, subsequently, correlate, via probability arguments, to an increase in the measure of entropy.
6.
Osmosisthe tendency of a fluid, usually water, to pass through asemipermeable membrane into a solution where the solventconcentration is higher, thus equalizing the concentrations ofmaterials on either side of the membrane.
7. Raoult's law is a phenomenological law that assumes ideal behavior based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules: the conditions of an ideal solution. This is analogous to the ideal gas law, which is a limiting law valid when the interactive forces between molecules approach zero, for example as the concentration approaches zero. Raoult's law is instead valid if the physical properties of the components are identical. The more similar the components are, the more their behavior approaches that described by Raoult's law. For example, if the two components differ only in isotopic content, then Raoult's law is essentially exact.
Comparing measured vapor pressures to predicted values from Raoult's law provides information about the true relative strength of intermolecular forces. If the vapor pressure is less than predicted (a negative deviation), fewer molecules of each component than expected have left the solution in the presence of the other component, indicating that the forces between unlike molecules are stronger. The converse is true for positive deviations.
Date: 2015-12-17; view: 1831
| <== previous page | | | next page ==> |
| Êîíòðîëüíàÿ ðàáîòà 3. | | | Culture Studies ROOM 507 |