
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
A Short Article on Articles
Many people believe that diamonds are formed from the metamorphism of coal. That idea continues to be the "how diamonds form" story in many science classrooms.
Coal has rarely played a role in the formation of diamonds. In fact, most diamonds that have been dated are much older than Earth's first land plants - the source material of coal! That alone should be enough evidence to shut down the idea that Earth's diamond deposits were formed from coal.
Another problem with the idea is that coal seams are sedimentary rocks that usually occur as horizontal or nearly horizontal rock units. However, the source rocks of diamonds are vertical pipes filled with igneous rocks.
Four processes are thought to be responsible for virtually all of the natural diamonds that have been found at or near Earth's surface. One of these processes accounts for nearly 100% of all diamonds that have ever been mined. The remaining three are insignificant sources of commercial diamonds.
These processes rarely involve coal.
1) Diamond Formation in Earth's Mantle
Geologists believe that the diamonds in all of Earth's commercial diamond deposits were formed in the mantle and delivered to the surface by deep-source volcanic eruptions. These eruptions produce the kimberlite and lamproite pipes that are sought after by diamond prospectors. Diamonds weathered and eroded from these eruptive deposits are now contained in the sedimentary (placer) deposits of streams and coastlines.
The formation of natural diamonds requires very high temperatures and pressures. These conditions occur in limited zones of Earth's mantle about 90 miles (150 kilometers) below the surface where temperatures are at least 2000 degrees Fahrenheit (1050 degrees Celsius) (1). This critical temperature-pressure environment for diamond formation and stability is not present globally. Instead it is thought to be present primarily in the mantle beneath the stable interiors of continental plates
Diamonds formed and stored in these "diamond stability zones" are delivered to Earth's surface during deep-source volcanic eruptions. These eruptions tear out pieces of the mantle and carry them rapidly to the surface (3), See Location 1 in the diagrams above and at right. This type of volcanic eruption is extremely rare and has not occurred since scientists have been able to recognize them.
Is coal involved? Coal is a sedimentary rock, formed from plant debris deposited at Earth's surface. It is rarely buried to depths greater than two miles (3.2 kilometers). It is very unlikely that coal has been moved from the crust down to a depth well below the base of a continental plate. The carbon source for these mantle diamonds is most likely carbon trapped in Earth's interior at the time of the planet's formation.
2) Diamond Formation in Subduction Zones
Tiny diamonds have been found in rocks that are thought to have been subducted deep into the mantle by plate tectonic processes - then returned to the surface (4). (See Location 2 in the diagrams above and at right.) Diamond formation in a subducting plate might occur as little as 50 miles (80 kilometers) below the surface and at temperatures as low as 390 degrees Fahrenheit (200 degrees Centigrade) (1). In another study, diamonds from Brazil were found to contain tiny mineral inclusions consistent with the mineralogy of oceanic crust. (8)
Is coal involved? Coal is a possible carbon source for this diamond-forming process. However, oceanic plates are more likely candidates for subduction than continental plates because of their higher density. The most likely carbon sources from the subduction of an oceanic plate are carbonate rocks such as limestone, marble and dolomite and possibly particles of plant debris in offshore sediments.
3) Diamond Formation at Impact Sites
Throughout its history, Earth has been repeatedly hit by large asteroids. When these asteroids strike the earth extreme temperatures and pressures are produced. For example: when a six mile (10 kilometer) wide asteroid strikes the earth, it can be traveling at up to 9 to 12 miles per second (15 to 20 kilometers per second). Upon impact this hypervelocity object would produce an energy burst equivalent to millions of nuclear weapons and temperatures hotter than the sun's surface (5).
The high temperature and pressure conditions of such an impact are more than adequate to form diamonds. This theory of diamond formation has been supported by the discovery of tiny diamonds around several asteroid impact sites. See Location 3 in the diagrams above and at right.
Tiny, sub-millimeter diamonds have been found at Meteor Crater in Arizona. Polycrystalline industrial diamonds up to 13 millimeters in size have been mined at the Popigai Crater in northern Siberia, Russia. Is coal involved? Coal could be present in the target area of these impacts and could serve as the carbon source of the diamonds. Limestones, marbles, dolomites and other carbon-bearing rocks are also potential carbon sources.
4) Formation in Space
NASA researchers have detected large numbers of nanodiamonds in some meteorites (nanodiamonds are diamonds that are a few nanometers - billionths of a meter in diameter). About three percent of the carbon in these meteorites is contained in the form of nanodiamonds. These diamonds are too small for use as gems or industrial abrasives, however, they are a source of diamond material (6), See Location 4 in the diagrams above and at right.
Smithsonian researchers also found large numbers of tiny diamonds when they were cutting a sample from the Allen Hills meteorite (7). These diamonds in meteorites are thought to have formed in space through high speed collisions similar to how diamonds form on Earth at impact sites.
Is coal involved? Coal is not involved in the creation of these diamonds. The carbon source is from a body other than Earth.
The Most Convincing Evidence The most convincing evidence that coal did not play a role in the formation of most diamonds is a comparison between the age of Earth's diamonds and the age of the earliest land plants. Almost every diamond that has been dated formed during the Precambrian Eon - the span of time between Earth's formation (about 4,600 million years ago) and the start of the Cambrian Period (about 542 million years ago). In contrast, the earliest land plants did not appear on Earth until about 450 million years ago - nearly 100 million years after the formation of virtually all of Earth's natural diamonds. Since coal is formed from terrestrial plant debris and the oldest land plants are younger than almost every diamond that has ever been dated, it is easy to conclude that coal did not play a significant role in the formation of Earth's diamonds. Contributor: Hobart King
A Short Article on Articles
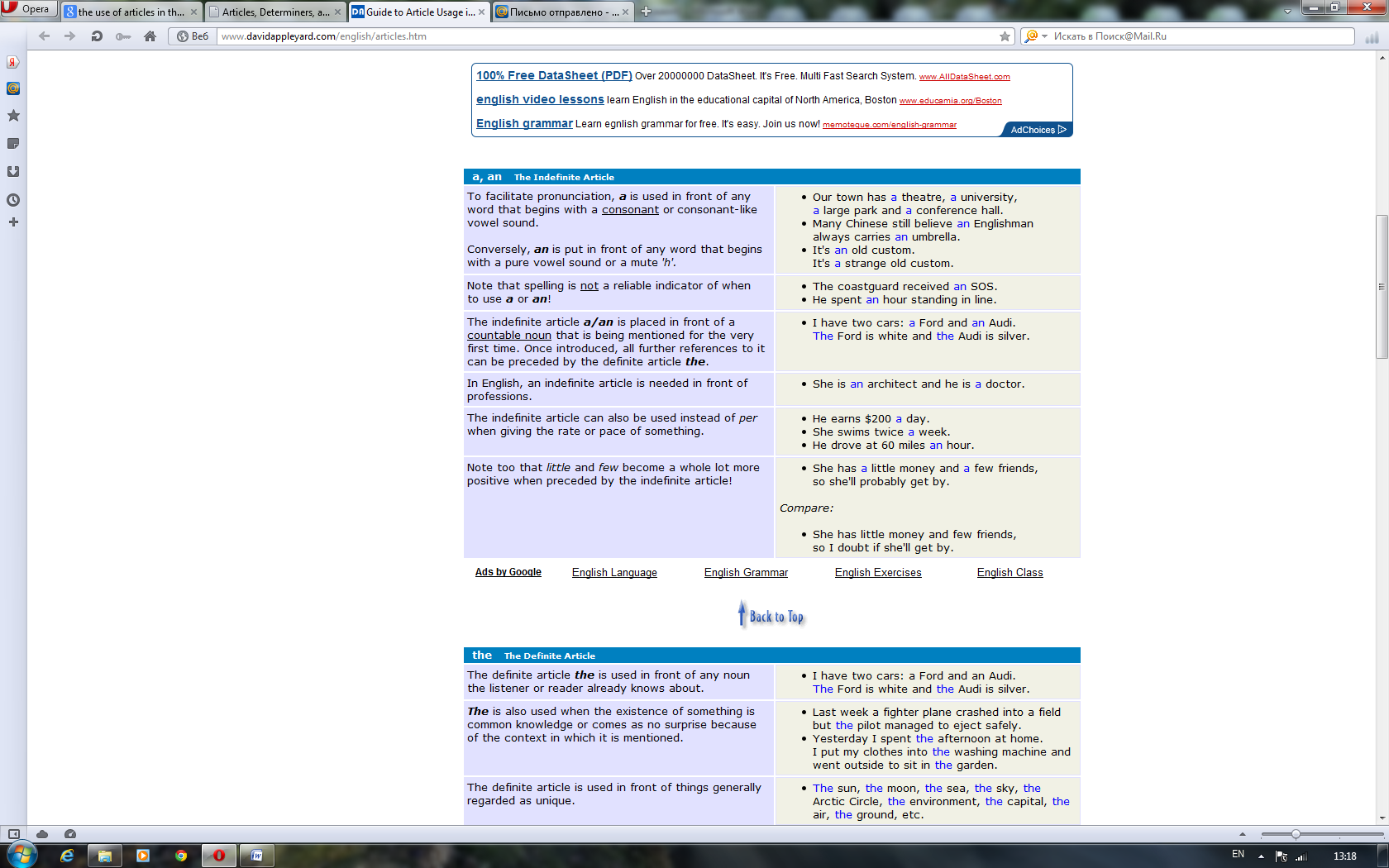
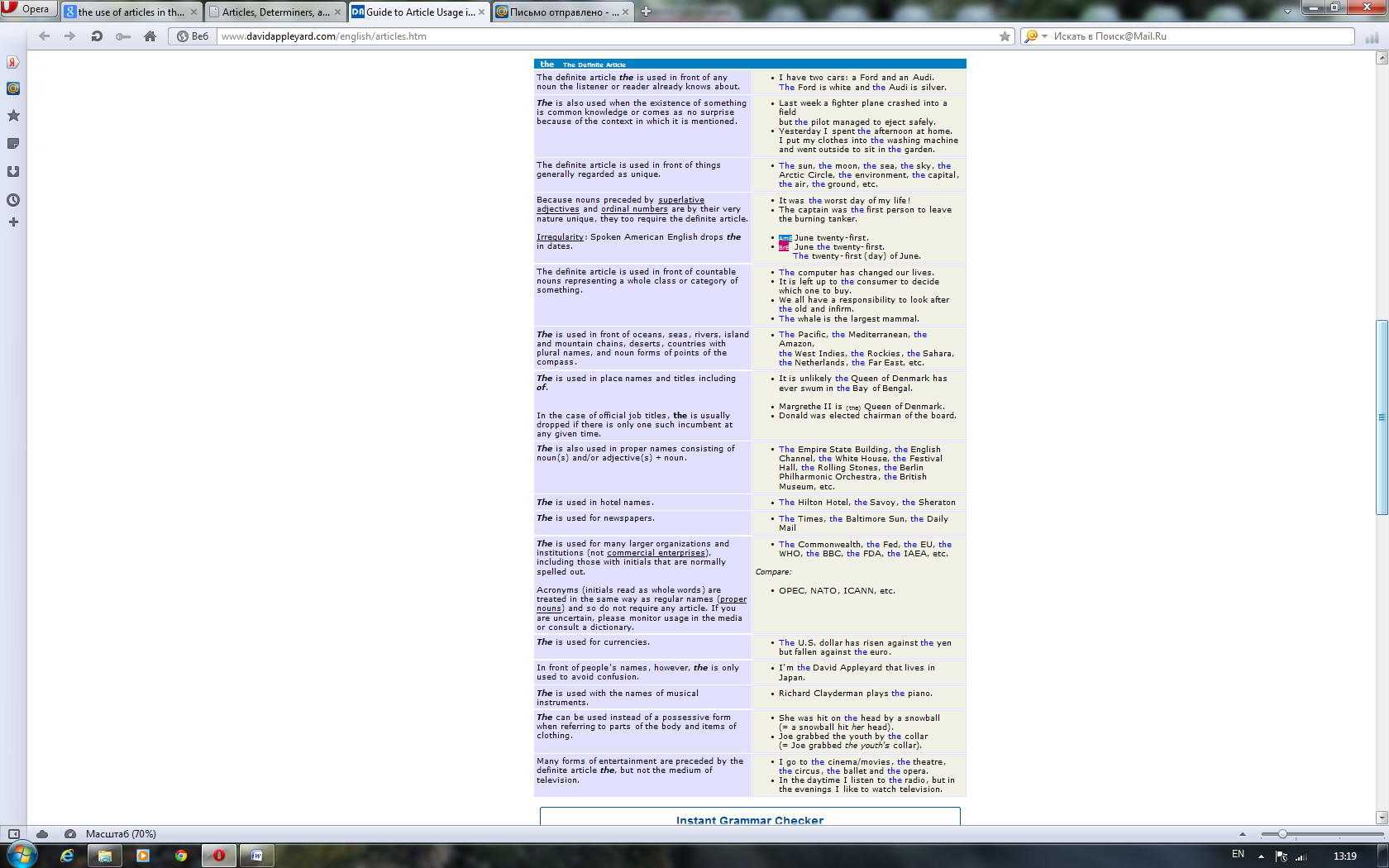
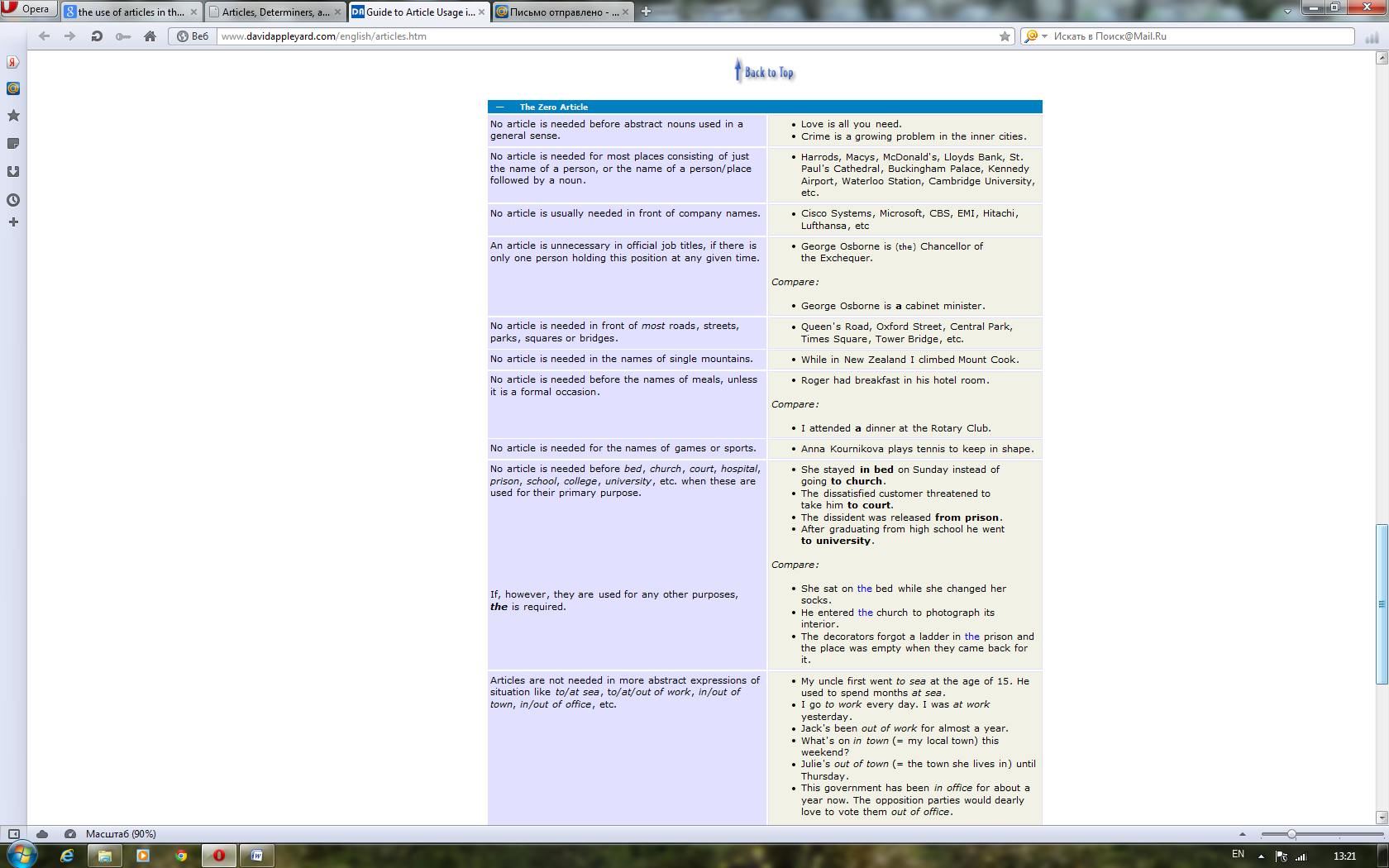
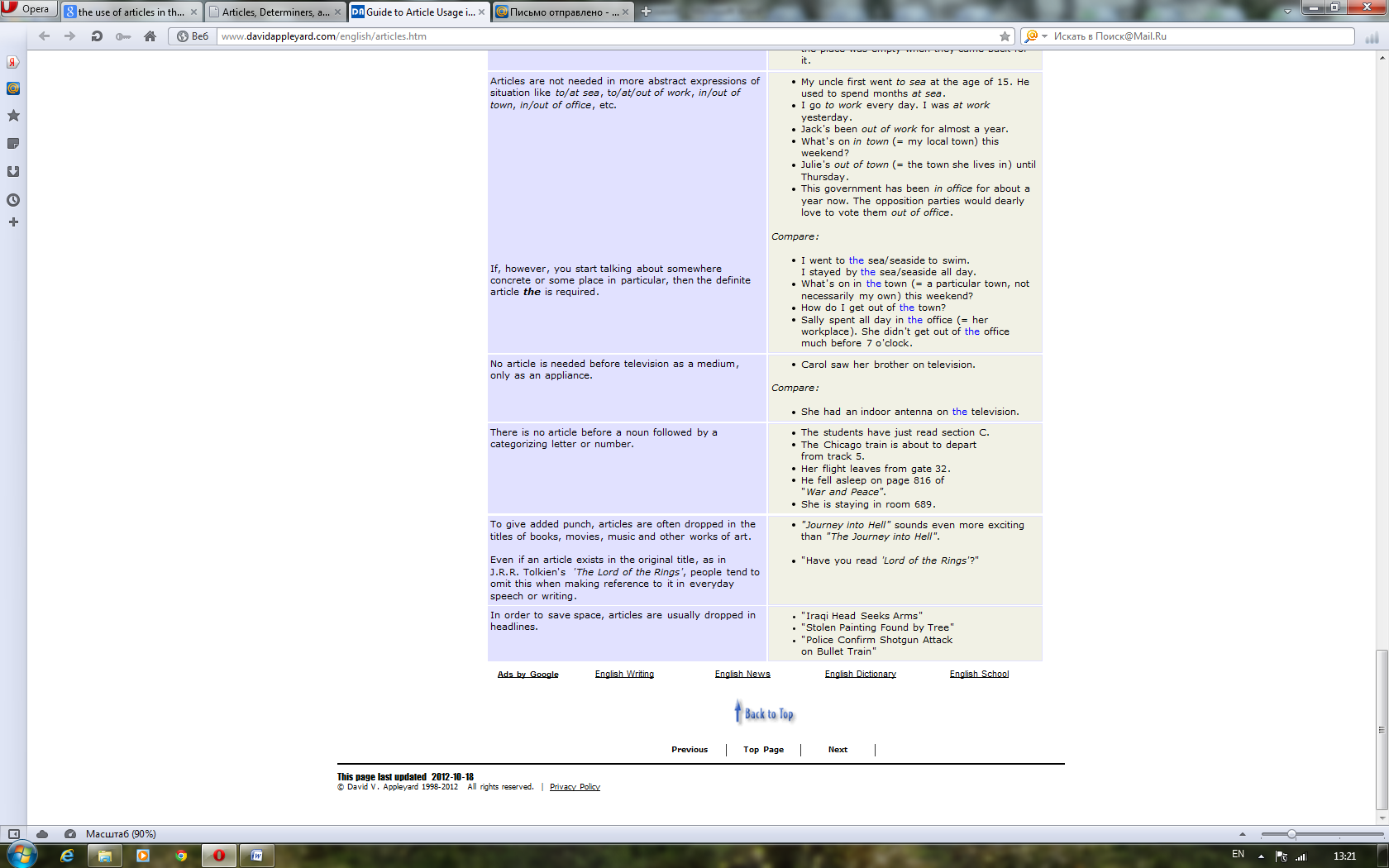
For better or for worse, English is blessed with articles. This causes a considerable amount of confusion for speakers of most of the world's other languages, who seem to get on rather well without them. The good news is that English began dropping the complex case systems and grammatical genders still prevalent in other European languages a very long time ago. Now we are left with just two forms of the indefinite article (a & an) and one form of the definite article (the). Perhaps more than anything it is the transition from being a language with synthetic structure to one which is more analytic that has helped gain English the kind of unrivalled worldwide acceptance it enjoys today.
Although greatly simplified, English article usage still poses a number of challenges to speakers of other European languages. Let's compare the German sentence "Da er Botaniker ist, liebt er die Natur" with the corresponding English one "Being a botanist, he is fond of nature". You'll see that English puts an indefinite article in front of a profession but German doesn't. Conversely, English manages without articles in front of abstract nouns like nature, where German needs a definite article.
Even between British and American usage one finds subtle differences in nuance or emphasis. For example, Americans usually say someone is in the hospital, much as they could be at the bank or in the park. To the British this sounds like there is only one hospital in town or that the American is thinking of one hospital in particular that he or she patronizes. The Brits say an ailing person is in hospital, just as they would say a child is at school or a criminal is in prison. This is because they are thinking more of the primary activities that take place within those institutions rather than the buildings in which they are housed. If, however, you are merely visiting one of these places, you are at the hospital, at the school or at the prison — both British and Americans agree here that what we have in mind is the building itself.
These few examples serve to illustrate that there is more to articles than at first meets the eye. From whatever perspective you are viewing this page, we hope you'll discover that articles are actually precision tools that greatly contribute to the unique accuracy of expression afforded by the English language. Most article usage does in fact have a reasonably logical explanation. If this can be properly grasped then non-native English can be made a lot less conspicuous and many misunderstandings avoided.
David V. Appleyard
Date: 2014-12-29; view: 1497
| <== previous page | | | next page ==> |
| Methods of Diamond Formation | | | How to Give Directions |