
Home
Random Page
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
|
Qualitative organic analysisThe first steps in the identification of organic compounds are based upon physical tests. These include appearance, color, odor, and solubility. These tests are followed by determining either the melting point (of solid substances) or the boiling point (of liquids). By comparing these results with those in published tables, the analyst can rapidly narrow down the number of chemical possibilities. Further identification then follows a well-established procedure. The elements present in the compound are determined by decomposing it into inorganic substances. It is then established whether the compound is an
 Flame testscan reveal the presence of various metals. A sample (preferably a chloride) is burned in a Bunsen flame on a platinum wire. The color of the flame identifies the metal. Na = sodium; Li = lithium; Sr = strontium; Ca = calcium; K = potassium; Ba = barium; Cu = copper; Pb = lead. Flame testscan reveal the presence of various metals. A sample (preferably a chloride) is burned in a Bunsen flame on a platinum wire. The color of the flame identifies the metal. Na = sodium; Li = lithium; Sr = strontium; Ca = calcium; K = potassium; Ba = barium; Cu = copper; Pb = lead.
|
|
|
|
|
|
|
|
| ■' ■■
| '..■ ■: ■
| | I
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Na
| Lij
|
| Sf
| Ca
|
| K
|
| Ba
|
| Cu
|
| Pb
|
| K +
Na
|
Analytical chemistry: Classical analysis 1Z9
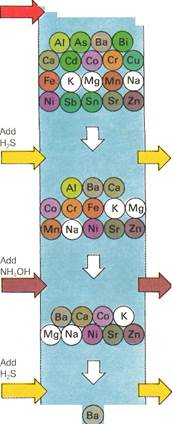
| Mixture containing up to 22 different metals
|
| Silver, mercury and lead as chlorides
|
| Arsenic, bismuth,
cadmium, copper, antimony and tin as sulfides
|
| Al^rYF.
Aluminum, chromium, iron and manganese as hydroxides
|
| (an
Cobalt, nickel and zinc as sulfides
|
| *3&
Barium, calcium and strontium as carbonates
|
acidic, alkaline, or neutral substance. Acids and alkalis are discussed in the article "Key chemical reactions" in the beginning of this book.
The preceding process is followed by a series of tests to determine the nature of the reactive groups in the compound. The tests are carried out on very small quantities of material. Many of these tests give rise to colored precipitates or solutions when a positive result is obtained. Once the substance has been provisionally identified, it may be confirmed by using one of a variety of other tests.
Date: 2015-12-11; view: 3167
|

 Flame testscan reveal the presence of various metals. A sample (preferably a chloride) is burned in a Bunsen flame on a platinum wire. The color of the flame identifies the metal. Na = sodium; Li = lithium; Sr = strontium; Ca = calcium; K = potassium; Ba = barium; Cu = copper; Pb = lead.
Flame testscan reveal the presence of various metals. A sample (preferably a chloride) is burned in a Bunsen flame on a platinum wire. The color of the flame identifies the metal. Na = sodium; Li = lithium; Sr = strontium; Ca = calcium; K = potassium; Ba = barium; Cu = copper; Pb = lead.
