
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Naphthalene and anthracene
Best-known and simplest of the condensed-ring hydrocarbons, naphthalene is a solid at room temperature. Like anthracene, also a solid at room temperature, it can be isolated from coal tar.
Products of both substances are used in the manufacture of dyestuffs and in the production of plastics and polyester resins. These resins are useful in the manufacture of paints, film, and synthetic fibers (polyester clothing). Certain compounds of naphthalene and anthracene, however, have been banned in many countries. An impurity present during the production of these compounds has been found to cause cancer.
Further condensed hydrocarbons can be made by fusing rings together. They can be added together to form a chain of rings, or joined honeycomb-fashion. Many useful products are derived from fusing rings together.
Coal carbonization is the major source of anthracene, naphthalene, and most other condensed aromatics. In the absence of air, coal is heated to a temperature of about 1650° F. (900° C). This causes the coal to break down into three main products—coal gas, coal tar, and coke. The coke is used in steel manufacture, and the gas as fuel gas. The other fraction, coal tar, contains many organic compounds, among them the condensed hydrocarbons. The most important compounds—benzene, naphthalene, and anthracene—are extracted using a combination of different techniques. Many other products are also contained in the coal tar, although in much smaller amounts.
Today, coal gas as a domestic heating fuel has given way in many places to natural gas. And less coke is required to smelt iron ore because blastfurnaces are more efficient. As a result, coal carbonization is declining. However, other sources of condensed hydrocar-
 Organic chemistry: Aromatic hydrocarbons 77
Organic chemistry: Aromatic hydrocarbons 77
 |  | ||||||||||||||||
 |  | ||||||||||||||||
 |  |  |  | ||||||||||||||
 |

|

|

|

|

|

|
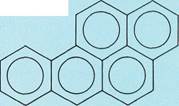
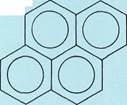
 Naphthalene
Naphthalene

Phenanthrene
Anthracen

Pyrene
Naphthacene

Benzapyrene
Condensed-ring aromatic compoundsconsist of two or more benzene rings joined together in chains of rings (top three examples to the left) or in honeycomblike arrangements (bottom three examples to the left). These structures are simply represented with all the carbon-carbon bonds being the same length, as in benzene. In fact, the distances between adjacent carbon atoms are not equal.
 bons are available. These include the exposing of gasoline to high temperatures.
bons are available. These include the exposing of gasoline to high temperatures.
Date: 2015-12-11; view: 3299
| <== previous page | | | next page ==> |
| Many types of cosmetics, | | | Cancer-causing properties |