
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Many types of cosmetics,
including theatrical makeup, contain ethylene glycols. These substances can be synthesized from the alkene ethene (ethylene, C2H4).
Red oil dropletsare floating off a fiber strand as a result of the action of a detergent in the liquid. Many detergents are synthesized using long-chain alkenes (having between 4 and 20 carbon atoms) as starting materials.

|
| i pi-electrons |
Benzeneis the simplest aromatic compound. It is usually the presence of at least one benzene ring that characterizes other compounds as aromatic. The structure of benzene is unusual. Instead of having alternate long single and shorter double carbon-carbon bonds in the six-membered ring, all the bonds are of equal length. The "spare" pi-electrons (that would otherwise be needed for the double bonds) are spread evenly round the ring. These pi-electrons comprise two doughnut-shaped clouds above and below it. Representations of the structure include the old Kekule formula (A) and the modern Robinson formula (B).
 Aromatic hydrocarbons
Aromatic hydrocarbons
 |

|
In oil refineries,such as the one shown below, a combination of fractional distillation and selective dissolving techniques is used to isolate aromatic hydrocarbons from their sources. Fossil fuels, principally oil and coal, are the chief sources of aromatic hydrocarbons.
All aromatic hydrocarbons are composed solely of carbon atoms and hydrogen atoms in various arrangements. What sets most of them apart from other hydrocarbons is the presence in their molecular structures of at least one benzene ring.
A benzene ring consists of six carbon atoms linked together in a planar hexagonal structure. This is a six-sided (hexagonal) structure, all of which is in one plane (planar). Each carbon atom of the benzene ring is bonded to a single hydrogen atom. The ability of chemists to replace these hydrogen atoms with other
groupings has given rise to the vast number of aromatic hydrocarbons we know today. Many also occur naturally in the coal and oil deposits that lie beneath the earth's crust. Others are widespread in nature itself.
Some of the most commonly used aromatic hydrocarbons include toluene and the xylenes. Every year chemists isolate millions of tons of these compounds from petroleum (crude oil) and, to a lesser extent, from coal. They are important industrially, especially as solvents. A solvent is a substance that is able to dissolve other substances. For example, gasoline is a solvent liquid able to dissolve grease spots. Aromatic hydrocarbons are also the starting materials for the manufacture of plastics. They are also used by laboratory chemists for conversion into other, more valuable compounds.
Benzene
The simplest aromatic compound is benzene itself, the building block from which aromatic chemistry sprang. Known since 1825, benzene is a colorless liquid at room temperature. It is one of the chief ingredients in coal tar. It can also be isolated from petrochemical feedstocks, such as naphtha. Naphtha, in turn, is derived from petroleum, coal tar, or natural gas. A feedstock is a principal material in any chemical process that produces petroleum products. There are various techniques for separating benzene and other aromatic hydrocarbons from coal, tar, and petrochemical feedstocks. These techniques include distillation (purifying) methods and the use of solvents to dissolve the required products selectively.
In the second half of the nineteenth century, the potential importance of products obtained from benzene became evident. Scientists noted the presence of the benzene nucleus in many naturally-occurring drugs and dyes. They then searched for ways to make them synthetically. But chemists investigating
 Organic chemistry: Aromatic hydrocarbons 75
Organic chemistry: Aromatic hydrocarbons 75

|

|
| The structuresof some of the simpler and better known aromatic hydrocarbons are illustrated below. As is apparent from the diagram, these substances consist of a single benzene ring with different groups attached. For example, there is a methyl group ( —CH3) in toluene and a hydroxyl group ( — OH) in phenol. |
| Styrene CH=CH„ |
| Chlorobenzene CI |
benzene were puzzled by its structure until as late as the 1920s. Many models were put forward to explain its molecular makeup. Nowadays, its structure has been rationalized using the concept of delocalized electrons.
Structures of most organic molecules are drawn in terms of single, double, or triple bonds between carbon atoms, each atom possessing the ability to form four bonds. But the properties and reactions of benzene do not conform with those of any single structure drawn in this conventional way. Instead, the six carbon atoms in benzene are considered to be held together by two-electron (single) bonds of equal length. The third electron in each carbon atom forms the bond with hydrogen. The fourth electron in each carbon atom (a total of six in the entire benzene ring) is not bonded with other electrons. These six electrons, which would otherwise be required for three double bonds, are evenly distributed around the hexagonal ring. These six delocalized electrons, known as pi-electrons, give rise to a doughnut-shaped electron cloud. This cloud is distributed above and below the plane of the ring atoms. As a result, all of the bonds between carbon atoms in benzene are the same length. They are shorter than a carbon-carbon single bond in a simple aliphatic (chainlike) compound, but longer than an ordinary carbon-carbon double bond.
The greater mobility of the pi-electrons accounts for the unique properties of benzene and its derivatives. For example, the reaction between bromine and a simple aliphatic al-kene, such as ethene (ethylene), is an addition reaction. The molecule of bromine attaches (adds) itself to the ethene. The reaction between bromine and benzene, however, is a substitution. One of the hydrogen atoms is replaced by a bromine atom.
Since its discovery, benzene has fulfilled
many roles industrially. It is an excellent solvent. It can also be converted into a vast range of derivatives—products derived (obtained) from a source—in this case, benzene. Styrene, a compound formed by adding benzene to ethene, helps in the making of polystyrene. Many other plastics, rubbers, and resins are built from benzene derivatives. It is also the basis of many substances essential to the synthetic chemist. Among the better-known benzene derivatives are nitrobenzene (used for making perfumes), aniline (for making dyes, certain medicines, and plastics), benzaidehyde (used in flavoring agents), and benzoic acid (used as an antiseptic and as a preservative).
Despite its undoubted usefulness, benzene must be treated with caution because it may cause cancer. As such, it has been banned from the classroom in most Western countries. It is used with great care in the laboratory and in industry.
|

|
| Aniline |
| Nitrobenzene |

|

|

|
Benzaidehyde CHO


|
This unusual buildingis a
house made almost entirely of polyurethane foam, a polymer derived from toluene. To build the house, a thick layer of the foam was sprayed on the inside of large hemispherical molds. The molds were removed after the foam had hardened. The exterior surface was then painted with sunscreen paint to block ultraviolet light, which would otherwise cause the polyurethane to break down. The interior surface was covered with a fire-proofing material, because polyurethane gives off poisonous fumes when burned.
76 Organic chemistry: Aromatic hydrocarbons
 |
| Orthoxylene or Metaxylene or 1,2-xylene 1,3-xylene | Paraxylene or 1,4-xylene | |||||
| 9H3 | X3 | -CH3 | 9H3 | |||
| '.''•'■' ■'•' ';vX;5ft*cK ? | - | |||||
| Xylenehas three isomeric forms. Orthoxylene has the second methyl group { —CH3), shown in yellow, in the ortho position, adjacent to the primary methyl (in black). In metaxylene, the second methyl (purple) is in the meta position, attached to a carbon atom two carbons away from the primary methyl. And in paraxylene, the second methyl (blue) is in the para position, opposite the primary methyl group. |
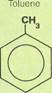
Toluene
A liquid at room temperature, toluene is identical to benzene except that one of the hydrogen atoms has been replaced with a methyl group. A methyl group is a molecule of methane without one of its hydrogen atoms. Thus, toluene is also called methylbenzene. Like benzene, from which it is easily derived, toluene is a good solvent, able to dissolve other substances. It is also used as a starting material for the high explosive TNT (trinitrotoluene), and for assorted plastics. In addition, toluene is used in the manufacture of preservatives (for cosmetics, beverages, and food), antiseptics, dyes, and perfumes. It is possible to convert toluene into a vast number of other derivatives. The major sources for toluene are coal tar and naphtha. It is extracted in the same way as benzene.
| The adhesiveunder test below consists principally of the polymer polychloro-prene (a synthetic rubber) dissolved in an aromatic solvent such as toluene. |
Xylenes

|
These are liquids at room temperature. They
are similar to toluene in structure, except that a further hydrogen atom has been displaced from the ring by a methyl group. Thus, they are also called dimethylbenzenes. Three types of xylene exist—orthoxylene, metaxylene, and paraxylene—depending on the positions of the two methyl groups about the ring. They are all useful solvents and are starting materials in the production of some types of products obtained from benzene. For example, paraxylene is the starting material for the synthetic fiber Terylene (Dacron). Again, they can be prepared from tars or naphtha using the same techniques as those for preparing benzene and toluene.
Date: 2015-12-11; view: 3425
| <== previous page | | | next page ==> |
| Reactions of unsaturated compounds | | | Naphthalene and anthracene |