
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Introduction to Polymer Science and Technology Behaviour of polymers
5.3.1.2 Composition of the backbone molecular chain
Structure of the repeating unit:
Backbone chains with aliphatic groups,e.g., - CH2- CH2- or the replacement of some of the "C" atoms by "O" (as in ether links) builds flexibilityinto a polymer, because of ease of rotation about these groups, and lowers T and T .
G m
Repeat-unit Polymer Tg, °C Tm, °C
-CH2-CH2- PE -120 130
CH3
-Si-O- Silicone rubber -123
(polydimethyl siloxane) CH,
Conversely, aromatic back-bone chains(i.e., benzene rings in the chain) cause stiffness,for example:
Introduction to Polymer Science and Technology Behaviour of polymers
| epoxy resins | |
| Poly-2,6-dimethyl | |
| phenylene oxide |
PEEK 143 334
PES (an amorphous TP) 230
Repeat-unit lengths and intermolecular forces:
The frequency of repeat-unit links, and the frequency and the type of intermolecular bonding (e.g., Van der Waals forces or H-bonds)influences T and Tm. In nylons, in spite of an aliphatic backbone, intermolecular H-bonds that are much stronger than Van der Waals forces hold neighbouring chains strongly together and also tend to stabilise the crystallites, therefore result in high T and Tm values. The melting temperature of nylons decrease as the frequency of H-bonding decreases, for example, the melting temperature of Nylon 4,6 (295 °C) > Nylon 6,6 (265 °C) > Nylon 6,10 (223 °C) > Nylon 10,10 (203 °C) , etc.
5.3.1.3 Side groups
| long flexiblealiphatic side groups decrease T and Tm. Rigid groups include aromatic and/or cyclic structures or tertiary |
Bulky,inflexible (rigid) side groups increase Òê and Tm by restriction of bond rotation and main chain stiffening, but long flexiblealij isomeric forms.
Repeat-unit PolymerTg,°C Tm,°C
H
I
-CH2-C- PP -10 170
^ (isotactic)

|
PS 100 240
(isotactic)
Introduction to Polymer Science and Technology
Behaviour of polymers
H I-CH2-C-
I
IH3C-C-CH3
I
CH3
polyvinyl t-butylether
polyvinyl iso-butylether
-18
I
ñí2
I
H3C-C-
I í
| CH | |
| CH | |
| CH | |
| CH |
polyvinyl n-butyl ether
-52
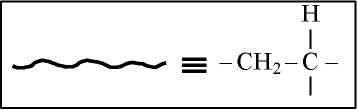
|
t - tert or tertiary
5.3.1.4 Molecular polarity
The presence ofpolargroupssuchas -Cl, -OH or -CN tends to raise T and Tm more than non-polar groups of equivalent size. The polar interactions restrict main-chain mobility further by promoting additional intermolecular forces.
Introduction to Polymer Science and Technology Behaviour of polymers
Repeat-unit PolymerTg,°C Tm,°C
H
I
CH2-C- PP -10 170
^ (isotactic)
H
I
-CH2-C- polyvinylalcohol 85 230
IOH
H
I
CH2-C- PVC 87(81) 227(273)
I
H
I
-CH2-C- polyacrylonitrile 104(130) 317
^ (syndiotactic)
5.3.1.5 Backbone molecular symmetry
Symmetry facilitates rotation about the backbone molecular chain and, thus, causes a drop in T and Tm.
Date: 2015-12-11; view: 1331
| <== previous page | | | next page ==> |
| Introduction to Polymer Science and Technology | | | Repeat-unit Polymer Tg,°C Tm,°C |