
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Introduction to Polymer Science and Technology
Microstructure
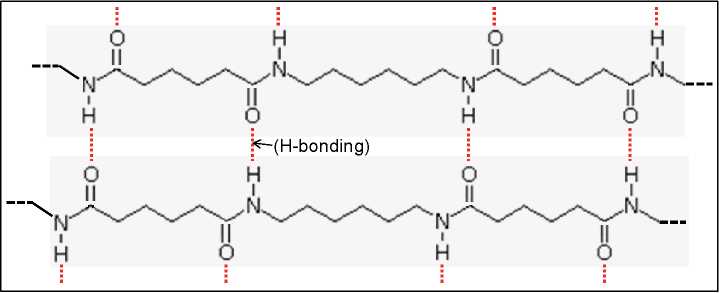
Figure 4.9H-bonding between the amide groups in polyfhexamethylene adipamide)
The degree of crystallinity indicates the amount of the material that is structurally crystalline, and it can be measured by a number of methods such as density, x-ray diffraction, infrared spectroscopy and thermal analysis. In using all these techniques for the determination of the degree of crystallinity, certain assumptions are made: (a) polymers consist of two separable phases - crystalline and amorphous, and (b) the properties of the phases are additive, i.e., the property of the material is the sum of the properties of the phases.
4.3.1 Density method
This is the easiest of the methods used, and density has become a convenient parameter to express crystallinity as in different grades of polyethylenes: LDPE, MDPE, HDPE, UHDPE, etc.
Introduction to Polymer Science and Technology
Microstructure
The method uses the rule of mixtures equation for the specific volumes of phases in the material:
V = xV
V
where, V is the specific volume, subscripts "c" and "a" represent crystalline and amorphous, and "x" is the mass fraction of crystalline phase.
Ë x = (V - V) / (V - V) or x = (Pc / p) [(p - Ðâ) / (Pc - Pa)]
where, p is the density of the polymer specimen for which the crystallinity is to be determined. The degree of crystallinitycan be determined as outlined below:
V can be determined by measuring the density of the given polymer, e.g., by using a density gradient column
or, more conveniently, a density balance.
Va from the totally amorphous form of the material (e.g., melt quenched in liquid nitrogen and its density
measured).
Vc can be determined by X-ray crystallography (i.e., crystalline unit cell dimensions are obtained by X-ray
measurements).
Calculation of V for PE:
Five polymer chains can be associated with the unit cell: four at the corners and one through the centre, see Figure 4.10.

Figure 4.10Illustration of PE unit cell
-CH2- group in Site-1 is shared by 8 unit cells, thus, the share of each unit cell is Vs (CH2). -CH2- group in Site-2 is shared by 4 unit cells, thus, the share of each unit cell is V4 (CH2). -CH2- group in Site-3 is shared by 8 unit cells, thus, the share of each unit cell is Vs (CH2).
Introduction to Polymer Science and Technology
Microstructure
.-. each corner of the unit cell contains Vi (CH2). i.e., 4 corners make up 2 (CH2) or one monomer unit.
By the same approach, the contribution of the central chain to the unit cell is also one monomer unit.
The volume occupied by 2 monomer segments = abc, therefore, by one ethylene molecule = (abc)/2, where, a, b and ñ
are the edge lengths of the unit cell.
Since there are N (Avogadros number) molecules in 1 mole, the volume occupied by 1 mole of the substance is N (abc/2). One mole of ethylene = 28 g, ä the volume occupied by lg of crystalline PE, Vc= N(abc) /56.
Density values for crystalline and amorphous components of various polymers are presented in Table 4.1. Table 4.1Density of some thermoplastics and their crystalline and amorphous phases
| polymer type | crystallinity, % | density, g/cm3 | ||
| crystalline | amorphous | typical | ||
| PA 66 | 35-45 | 1.24 | 1.07 | 1.14 |
| PA 6 | 35-45 | 1.23 | 1.08 | 1.14 |
| POM | 70-80 | 1.54 | 1.25 | 1.41 |
| PET | 30-40 | 1.5 | 1.33 | 1.38 |
| PBT | 40-50 | - | - | 1.3 |
| PTFE | 60-80 | 2.35 | 2.1 | |
| i-PP | 70-80 | 0.95 | 0.85 | 0.905 |
| a-PP | 50-60 | 0.95 | 0.85 | 0.896 |
| HDPE | 70-80 | 0.85 | 0.95 | |
| LDPE | 45-55 | 0.85 | 0.92 |
4.3.2 X-ray method
X-ray diffraction/scattering is used for polymers to characterise them in terms of their crystalline and amorphous states. The method is appropriate because X-ray diffraction/scattering produces different patterns by amorphous and crystalline regions of a polymer: a well-identifiable characteristic pattern (sharp peaks on the intensity vs. diffraction angle trace, see Figure 4.11) when scattered by the crystalline region, whereas an indistinct/diffused pattern (the amorphous halo) by the amorphous matter.
Date: 2015-12-11; view: 931
| <== previous page | | | next page ==> |
| Introduction to Polymer Science and Technology | | | Introduction to Polymer Science and Technology |