
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Discussion
The magnetotactic bacteria are the most primitive organisms known which use a vacuole-based system to form their biomineral products. In mammals, much of the
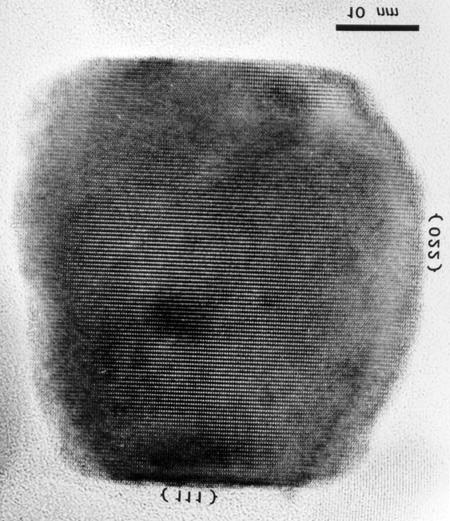
Figure 10.5. Magnetite crystal extracted from tissues of the human brain [58].
hydroxyapatite in bone and teeth is formed via a similar process, in which the chemical precursors are transported first to a vacuole storage system, and then dis-crete peptides are added to nucleate the desired crystal forms [9]. Understanding how this process evolved in bacteria ought to provide insights for understanding how the more complex biomineralization systems in eukaryotes operate.
Most of the published genetic analyses of magnetotactic bacteria to date have focused in using 16s RNA to infer phylogenetic relationships [74-77]. As of this writing, only one protein directly involved in the magnetite biomineralization process has been found using transposon mutagenesis [78], in an as-yet unnamed magnetotactic bacterium dubbed AMB-1. The protein coded by this open reading frame (termed MagA) is known to reside both in the cell membrane and in the magnetosome membrane [79], and is involved in transporting and accumulating iron within the magnetosomes [80]. It demonstrates strong homology with known Ca2+ trans-membrane transport proteins in other bacteria.
In an attempt to attack the magnetosome problem from the standpoint of the iron mediating enzymes, Bertani et al. [81] focused on the gene coding for bacter-ioferritin (bfr) in Magnetospirillum magnetotacticum. In contrast to E. coli, which has only one bfr gene, M. magnetotacticum has two genes, bfr1 and bfr2, which are strongly homologous to bacterioferritins. The subunit encoded by bfr1 is more similar to E. coli bacterioferritin than it is to the subunit encoded by bfr2. These genes are strange in two other ways: First, the open reading frames overlap by one base pair, with the last base pair of the stop codon of the first gene serving as the first codon of the second protein. Second, the amino acid residues of the region in the putative bfr2 subunit, which is thought to be involved in the binding and nucleating of the iron oxide mineral at the core of the ferritin protein (the mineral ferrihydrite), are completely different from the other bacterioferritins, which are otherwise highly conserved. This characteristic indicates that the proteins are not acting to nucleate ferrihydrite deposition, and Bertani et al. [81] speculate that this peculiar feature may have something to do with the mineralization process.
In summary, the genetic basis of biomineralization – for all mineral systems – is still a mystery. The “Grand Unified Theory of Biomineralization” presented here suggests that an understanding of magnetite biomineralization in the magnetotactic bacteria might provide a template for unraveling, or at least understanding, por-tions of vacuolar-based biomineral systems in higher animals, including humans. The first major step for understanding the bacterial system would be, of course, determining the complete genome sequence for a magnetotactic bacterium.
It is fitting to close this article with the first stanza of Rudyard Kipling’s famous poem, Cold Iron:
Gold is for the mistress – silver for the maid – Copper for the craftsman cunning at his trade. "Good!" said the Baron, sitting in his hall,
"But Iron – Cold Iron – is master of them all."
Date: 2015-02-03; view: 1368
| <== previous page | | | next page ==> |
| Magnetite Biomineralization | | | References |