CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
DEVELOPMENT AND BIOLOGY OF BRADYZOITES AND TISSUE CYSTS IN VIVOHistoryLainson (104) reviewed earlier literature on the development of tissue cysts. Levaditi et al. (107) apparently were the first to report that T. gondii may persist in tissues for many months as “cysts.” However, considerable confusion between the terms “pseudocysts” (group of tachyzoites) and “cysts” existed for many years. Frenkel and Friedlander (77) and Frenkel (70) cytologically characterized cysts containing organisms with a subterminal nucleus and PAS-positive granules surrounded by a argyrophilic cyst wall. Wanko et al. (177) first described the ultrastructure of the T. gondii cysts and its contents. Lainson (104) provided evidence that cysts were formed in mice as early as 8 days after the inoculation of tachyzoites. His illustrations of a cyst with four organisms on day 8 postinfection (p.i.) and with 20 organisms on day 10 p.i. provided the first convincing evidence of young cysts of T. gondii. Older cysts were up to 60 μm in diameter and contained approximately 3,000 organisms. Jacobs et al. (93) first provided a biologic definition of cysts when they found that cystic organisms were resistant to digestion by gastric juice (pepsin-HCl) whereas tachyzoites were destroyed immediately. Thus, cysts became important in the life cycle of T. gondii because carnivorous hosts could become infected by ingesting cysts. When T. gondii oocysts were discovered in cat feces in 1970 (27, 72, 75), oocyst shedding was added to the biologic definition of the cyst. Dubey and Frenkel (48) made the first in-depth study of the development of the tissue cysts and bradyzoites and defined cysts biologically and morphologically. They found that the cysts were formed as early as 3 days after inoculation of mice with tachyzoites. Cats shed oocysts with a short prepatent period (3 to 10 days) after ingesting bradyzoites, whereas after they ingested tachyzoites or oocysts, the prepatent period was longer (≥14 days). Ferguson and Hutchison (65) reported the first in-depth ultrastructural studies of the development of T. gondiicysts. Dubey and Beattie (45) proposed that cysts should be called tissue cysts to avoid confusion with oocysts. Host cells parasitized and prevalence. T. gondiitissue cysts occur in many organs and cell types. Most observations have been made with tissue cysts in the brains of mice, but the acid-pepsin digestion procedure and bioassay in cats have shown that tissue cysts occur in many extraneural organs. Tissue cyst distribution is in part controlled by the host and strain of T. gondii. The distribution of tissue cysts in different organs of animals fed the GT-1 strain ofT. gondii is shown in Table3. In pigs, dogs, cats, and rodents fed oocysts of the VEG strain of T. gondii, more tissue cysts were found in muscular tissues than in the brain in cats, dogs, and pigs whereas more tissue cysts were found in the brain than in other organs in rats and mice (38-40, 49, 110). Infections withT. gondii in experimental animals appear to be essentially the same as in some naturally infected animals as reviewed by Dubey and Beattie (45). For example, Jacobs et al. (92) isolated T. gondii from 18 diaphragms and 9 brains of 31 seropositive sheep killed at a slaughterhouse in New Zealand. View this table: TABLE 3. Persistence of T. gondii in tissues of animals fed the GT-1 straina
· ↵a Pepsin digests (50 g) were bioassayed in mice. · ↵b ND, not done. Size of tissue cysts and numbers of bradyzoites.Tissue cyst size is dependent on cyst age, the type of host cell parasitized, and the cytological method used for measurement. Young tissue cysts may contain as few as two bradyzoites surrounded by a distinct cyst wall and measure about 5 μm in diameter (Fig. 11B). Tissue cysts in myocytes are two to three times longer than those in neural cells (36). Because tissue cysts are most numerous in the brains of mice, most observations were made with neural tissue cysts. Beverley (4) measured the volume of tissue cysts liberated from the mouse by grinding brains with a mortar and pestle in saline (0.85% NaCl). Tissue cysts grew uniformly up to 10 weeks, after which there was considerable variability in tissue cyst size, perhaps due to a second generation of tissue cysts. The tissue cysts were up to 58 μm in diameter. He also stated that a tissue cyst may contain 60,000 organisms. Van der Waaij (174) made an in-depth study of the growth of tissue cysts in mouse brains in which 100 to 500 free (liberated from mouse brains by homogenization in saline), unstained tissue cysts were measured in the brains at 4, 8, 12, 16, and 24 weeks from mice inoculated subcutaneously (s.c.) with tissue cysts. The tissue cysts grew uniformly in size up to 12 weeks p.i., and then the growth levelled off. The tissue cysts were up to 70 μm in diameter, but the mean diameter of 100 tissue cysts at 16 weeks p.i. was 42 μm. Ferguson and Hutchison (65) measured tissue cysts in thin (≤1-μm) sections from mouse brain fixed in glutaraldehyde. They ultrastructurally observed 140 tissue cysts with a minimum of 10 tissue cysts at 11, 21, and 28 days and 3, 6, 12, 18, and 22 months. The tissue cysts were up to 20 μm in diameter at 28 days, up to 30 μm at 3 months, and up to 50 μm at ≥6 months. Dubey (38) measured tissue cysts in the brains of rats 72 to 75 days after feeding the rats oocysts. The brains were fixed in 10% buffered neutral formalin and sectioned at 5-μm thickness. Although the tissue cysts (n = 224) were up to 50 μm in diameter, most of them were approximately 30 μm in diameter. In addition to these reports, Dubey (44a) has never seenT. gondii tissue cysts larger than 70 μm in diameter in formalin-fixed paraffin-embedded sections of brains of hundreds of naturally or experimentally infected animals. The measurement of tissue cysts in formalin-fixed, paraffin-embedded sections provides a standard means of reporting results. The sizes of the tissue cysts vary a great deal when unstained tissue cysts are examined between a glass slide and coverslip, depending on the homogeneity of the brain suspension, the amount of fluid, and the pressure applied. A highly flattened tissue cyst that initially passed through a 63-μm filter is shown in Fig.26.
View larger version: FIG. 26. A highly stretched tissue cyst estimated to contain more than 1,000 bradyzoites in an impression smear of brain homogenate from a rat 14 months after infection with the VEG strain of T. gondii. The cyst wall (arrow) is barely visible. The size of the T. gondii tissue cyst may vary with the strain of T. gondii. Although there are no firm data, one of us (44a) has observed up to 300% variability in tissue cyst size at 2 months after oral inoculation of mice with oocysts of different isolates of T. gondii. The tissue cysts of some isolates were only 20 μm in diameter, whereas others were up to 60 μm in diameter. There are no firm data on the number of bradyzoites in a tissue cyst. Most of the information is from reviews (4, 137). In a large, flattened tissue cyst illustrated by Huskinson-Mark et al. (91), 990 bradyzoites are clearly visible. The 60,000 bradyzoites in a tissue cyst mentioned by Beverley (4) appears unrealistic. Separation of tissue cysts from host tissue.Cornelissen et al. (21) described a method to separate tissue cysts from the brains of mice after suspensions of brain homogenates were run on discontinuous Percoll gradients. According to these authors, T. gondii tissue cysts have a specific gravity of 1.056. This method has been widely used. Tissue cysts can also be separated on discontinuous gradients with 25 to 30% Percoll (7, 132) or a 20% dextran solution (79). The degree of success in purifying tissue cysts depends on the host tissue and the amount of blood contamination. To minimize tissue and erythrocyte contamination, the mice should be bled out before the tissue is harvested and the brain homogenate should be passed through a 90-μm wire sieve. Tissue cysts ofT. gondii are smaller than 90 μm and pass through the sieve. They can be stored in saline at 4°C for 2 months (41, 93). However, the mortality rate of bradyzoites stored at 4°C for prolonged periods has not been determined. Genetic regulation of tissue cyst numbers.Mice are often used to obtain tissue cysts of T. gondii for experimental purposes, and the most frequently used T. gondii strains are Beverley and ME-49. Tissue cyst numbers in mouse brains vary depending on the strain of mouse, the strain ofT. gondii, the route of inoculation, and the number of organisms inoculated. More tissue cysts are produced if mice become mildly ill but without obvious clinical signs. With the original Beverley strain, outbred mice inoculated s.c. with tissue cysts developed mild illness (weight loss during weeks 2 and 3) but survived. Hundreds of large tissue cysts were seen when the mice were killed at 12 weeks p.i. However, this strain has been passaged frequently in mice and has become more pathogenic for mice. Similarly, the pathogenicity of some lines of the ME-49 strain has increased since its original isolation. Therefore, to prevent mortality, one may have to use prophylaxis (sulfadiazine sodium or sulfamerazine in drinking water at 15 to 100 mg/100 ml of water) when the mice become ill. The dosage and duration of chemotherapy should be adjusted for each T. gondii strain, stage of the parasite inoculated, and strain of mice; there is no standard formula. The number of tissue cysts in mouse brain is genetically regulated (6, 16-18,117). Brown et al. (17) reported that the tissue cyst burden was regulated by mouse chromosome 17 containing the class I gene Ld . More tissue cysts were produced in congenic mice. Tissue cyst persistence may vary with the duration of infection, depending on the strain of T. gondii and the host. In one experiment with the ME-49 strain, the number of tissue cysts recovered from the brains of CBA/Ca mice at 4, 8, 12, and 16 weeks p.i. were 3,720, 2,158, 3,133, and 1,538, respectively (62). Therefore, the optimal time of harvest should be determined for each strain of T. gondii in a given host. Suzuki et al. (169) compared tissue cyst formation and mortality in two inbred strains of mice inoculated intraperitoneally (i.p.) with tissue cysts of the ME-49 strain. At 3 weeks after inoculation of 20 tissue cysts or more, nearly 10 times as many tissue cysts were found in the brains of CBA/Ca mice, all of which survived, as compared with BALB/c mice, two of which had died. After i.p. inoculation with 80 tissue cysts, the mortality rate during acute infection increased to 75% in BALB/c mice, whereas all CBA/Ca mice survived. However, during chronic infection, 50% of CBA/Ca mice died between 2 and 6 months p.i. whereas all BALB/c mice survived. Therefore, the optimal time to harvest tissue cysts may vary with the strain of T. gondii and the strain of the mouse. Tissue cyst rupture and reactivation of latent infection.It is well known that T. gondii tissue cysts persist in organs of infected hosts for several months and perhaps for life, depending on the host and parasite strains. The localization of tissue cysts also varies with the host and the strain of T. gondii. Although more tissue cysts are found per gram of tissue in mice than in other hosts, the fate of tissue cysts is difficult to study in mice because mice are never completely immune to T. gondii and new tissue cysts (Fig. 12) are formed even in chronic infections (4, 46, 62, 174). Even tachyzoites are present in the brains of chronically infected mice (52, 62). Because T. gondii tissue cysts are small and tissue cyst rupture is unpredictable, Frenkel (70) studied tissue cyst rupture in hamsters by using T. gondii and a related parasite,Besnoitia jellisoni, where intact tissue cysts were not associated with inflammatory reaction. Leaking and ruptured tissue cysts were accompanied by marked inflammation. Microglial nodules were common in chronically infected hamsters, and in some T. gondiiantigen could be shown to be present. He proposed that some tissue cysts rupture in chronically infected animals and that the released bradyzoites are destroyed by the immunocompetent host. However, in immunosuppressed animals, the released bradyzoites are believed to reactivate T. gondii infection. The factors affecting tissue cyst rupture are largely unknown. T. gondii tissue cyst rupture has been documented only rarely. Ferguson et al. (66) quantitatively studied tissue cyst rupture in mice chronically infected with the STR strain of T. gondii. Only 2 (0.27%) ruptured tissue cysts were seen among 750 tissue cysts examined, although T. gondii-positive debris was found in eight (1.4%) glial nodules. Tissue cyst rupture was documented in a Panamanian night monkey (Aotus lemurinus) that had been vaccinated three times with an attenuated (ts-4) strain of T. gondii and then challenged orally with tissue cysts of a complete T. gondii strain (55, 76). Glial nodules were found around degenerating tissue cysts. Degenerating tissue cysts were found in rats inoculated orally with oocysts of the VEG strain in the absence of tachyzoites or formation of new tissue cysts (38). The mechanism of formation of new generations of tissue cysts in chronically infected mice is unknown (Fig.27 to29). Clusters of tissue cysts are found in mice infected with certain strains of T. gondii(163), sometimes in clinically normal mice (Fig. 28). Whether bradyzoites leak from intact tissue cysts is uncertain (174). The rupture of tissue cysts and subsequent multiplication of tachyzoites can lead to fulminating toxoplasmosis even in chronically infected mice (Fig.29).
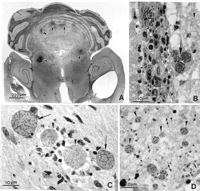
View larger version: FIG. 27. Section of the cerebrum of a patient with AIDS. Note the large area of necrosis (large arrow) and several small satellite areas (small arrows), probably due to tissue cyst rupture and subsequent growth of tachyzoites. Intact tissue cysts (arrowheads) are also present. All the black dots areT. gondii. Immunohistochemical stain with anti-T. gondii serum.
View larger version: FIG. 28. Cluster of T. gondii tissue cysts in the brain of a mouse which in life showed no apparent clinical signs. Unstained squash smear.
View larger version: FIG. 29. Encephalitis in the brain of a mouse 87 days after being fed oocysts of the VEG strain of T. gondii. Hematoxylin and eosin stain. (A) Coronal section showing grossly visible areas of necrosis (arrows) in the cerebellum and pons. (B) Necrosis (arrowhead) in the cerebrum and numerous tachyzoites (small arrows) in a meningeal blood vessel with severe meningitis and a group of extravascular tachyzoites (large arrow). (C) Four tissue cysts, two of which (arrowheads) are degenerating. (D) Numerous tissue cysts (arrowheads) and tachyzoites (arrows). Little information about the mechanisms of relapse is available. Treatments with corticosteroids, antilymphocyte serum, and anti-interferon (IFN) antibody are known to induce immunosuppression and relapse due to toxoplasmosis (3, 78,81, 82, 119, 126, 162,165-167). The animal model, route of inoculation and stage ofT. gondii used for primary infection, and criteria used for evaluation of relapses are all important considerations. For example, hamsters are more corticosteroid sensitive than are mice or rats, and orally administered corticosteroids are less effective than parenterally administered corticosteroids (74). Chronically infected hamsters initially immunized with the RH strain died of overwhelming toxoplasmosis following corticosteroid treatment (78). Similar relapsing fatal toxoplasmosis has not been induced in mouse models. Odaert et al. (127) examined the brains of chronically infected mice 6, 9, and 12 days after oral administration of dexamethasone. They reported seeing more foci of necrosis and more tissue cysts in cortisone-treated mice than in controls. However, there was no convincing evidence that relapse had occurred in this short period following corticoid administration (74). In experiments involving chronically infected mice, Sumyuen et al. (165) found that there was an increased mortality rate after immunosuppressive therapy (cortisol acetate or azathioprine, or azathioprine plus cortisol acetate) but that the number of T. gondii organisms in the brains and lungs of drug-treated mice remained similar to that found in untreated mice. Similar results were reported recently by Nicoll et al. (126), who found increased numbers of necrotic and gliotic foci, but no increase in parasite numbers, in dexamethasone-treated and chronically infected T. gondii mice. However, many tachyzoites die in the necrotic foci and do not infect new cells. Miédougé et al. (119) compared the clinical course and parasite numbers in mice injected i.p. with tissue cysts of the Beverley strain of T. gondii. Starting 50 days p.i., group A and B mice were injected weekly with anti-IFN rabbit antiserum and group C were controls. Group B mice also received antitoxoplasmic drugs (pyrimethamine and sulfadiazine in drinking water). On day 68 (i.e., 18 days after treatment), five mice from each group were killed and homogenates of lungs and brains were bioassayed in tissue culture. All the mice remained clinically normal. The T. gondiinumbers were dramatically higher in group A mice. All five mice in group A had 856 to 1,337 T. gondii organisms/g of lung, whereas only one of five mice in group B and no mice in group C had organisms. Because histologic examination was not performed, the results may be misleading because rupture of a single tissue cyst while making tissue homogenates for bioassay can result in a ≥1,000-fold difference in T. gondii infectivity titers. The authors also reported that there was parasitemia 53, 61, and 68 days after infection even in mice not given anti-IFN antibody. Thus, the mouse model does not appear to be an accurate representation for relapsing toxoplasmosis in AIDS patients (3). One should also take into account that clusters of tissue cysts of different sizes, numbers of tachyzoites, and reactivated lesions occur in chronically infected, nonimmunosuppressed mice (52). Although it has been known for 30 years that immunity to T. gondii is cell mediated (71), the precise mechanism of relapse is unknown. Clinical toxoplasmosis in AIDS patients is thought to be due to reactivation of a chronic infection, probably mediated by CD4+ lymphocyte deficiency (82). Production of the cytokine IFN-γ in mice is considered to be the main mediator of immunity to toxoplasmosis in both acute and chronic infection (167, 168). Mice chronically infected with the ME-49 strain of T. gondii died 10 to 18 days after administration of anti-IFN antibody, and depletion of both CD4+ and CD8+lymphocytes was needed to induce mortality in chronically infected mice (82). Depletion of CD8+ lymphocytes alone induced mortality in some mice, but mortality was not induced in those depleted of only CD4+lymphocytes. Lesions, similar to those seen in AIDS patients, were seen in mice that died after the administration of anti-IFN antibody; they consisted of necrosis and infiltration of neutrophils associated with the presence of tachyzoites (82). Because a few tachyzoites might be present even in chronically infected asymptomatic mice, it remains to be determined whether the reactivation is initiated by these “dormant” tachyzoites or bradyzoites released from tissue cysts. Date: 2016-01-03; view: 847
|