
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Previous SectionNext Section
Structures of Toxoplasma gondiiTachyzoites, Bradyzoites, and Sporozoites and Biology and Development of Tissue Cysts
1. J. P. Dubey1,*,
D. S. Lindsay2, and
C. A. Speer3
+Author Affiliations
- Parasite Biology and Epidemiology Laboratory, Livestock and Poultry Sciences Institute, USDA Agricultural Research Service, Beltsville, Maryland 20705-23501;
- Center for Molecular Medicine and Infectious Diseases, Virginia Tech, College of Veterinary Medicine, Blacksburg, Virginia 240612; and
- Veterinary Molecular Biology, Montana State University, Bozeman, Montana 597173
SUMMARY
Infections by the protozoan parasite Toxoplasma gondii are widely prevalent worldwide in animals and humans. This paper reviews the life cycle; the structure of tachyzoites, bradyzoites, oocysts, sporocysts, sporozoites and enteroepithelial stages of T. gondii; and the mode of penetration of T. gondii. The review provides a detailed account of the biology of tissue cysts and bradyzoites including in vivo and in vitro development, methods of separation from host tissue, tissue cyst rupture, and relapse. The mechanism of in vivo and in vitro stage conversion from sporozoites to tachyzoites to bradyzoites and from bradyzoites to tachyzoites to bradyzoites is also discussed.
Infections by the protozoan parasiteToxoplasma gondii are widely prevalent in humans and animals worldwide. T. gondii has emerged as one of the most common opportunistic infections in patients with AIDS. Toxoplasmosis in AIDS patients is considered to be a result of reactivation of latent infection, but the mechanisms of reactivation are unknown. This review focuses on the structure and biology of T. gondii stages (tachyzoites, bradyzoites, and tissue cysts) in intermediate hosts (humans) and the resistant (oocyst) stage outside the host.
Previous SectionNext Section
BASIC STRUCTURE AND LIFE CYCLE
There are three infectious stages of T. gondii: the tachyzoites (in groups or clones), the bradyzoites (in tissue cysts), and the sporozoites (in oocysts). These stages are linked in a complex life cycle (Fig. 1).
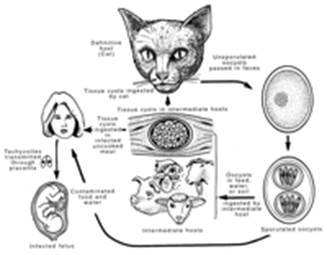
View larger version:
FIG. 1.
Life cycle of T. gondii.
TachyzoitesThe term “tachyzoite” (tachos = speed in Greek) was coined by Frenkel (73) to describe the stage that rapidly multiplied in any cell of the intermediate host and in nonintestinal epithelial cells of the definitive host. The term “tachyzoite” replaces the previously used term “trophozoite” (trophicos = feeding in Greek). Tachyzoites have also been termed endodyozoites or endozoites. Aggregates of numerous tachyzoites are called clones, terminal colonies, or groups.
The tachyzoite is often crescent shaped, approximately 2 by 6 μm (Fig. 2), with a pointed anterior (conoidal) end and a rounded posterior end. Ultrastructurally, the tachyzoite consists of various organelles and inclusion bodies including a pellicle (outer covering), apical rings, polar rings, conoid, rhoptries, micronemes, micropore, mitochondrion, subpellicular microtubules, endoplasmic reticulum, Golgi complex, ribosomes, rough and smooth endoplasmic reticula, micropore, nucleus (Fig. 2 to 10), dense granules, amylopectin granules (which may be absent), and a multiple-membrane-bound plastid-like organelle which has also been called a Golgi adjunct or apicoplast (24-26, 103, 145,176). The nucleus is usually situated toward the central area of the cell and contains clumps of chromatin and a centrally-located nucleolus.


View larger version:
FIG. 2.
Tachyzoites of T. gondii. A dividing tachyzoite (arrowheads) and single tachyzoites (arrows). Impression smear feline lung, stained with Giemsa stain.
The pellicle consists of three membranes, a plasmalemma and two closely applied membranes that form an inner membrane complex (Fig.3 and 4) which is formed from a patchwork of flattened vesicles (121, 136,145, 176). The inner membrane is discontinuous at the anterior tip above the polar rings, at micropores which are situated laterally, and at the posterior pore at the extreme posterior tip of the zoite. Polar ring 1 is an electron-dense thickening of the inner membrane complex at the anterior end of the tachyzoite, which encircles a cylindrical, truncated cone called the conoid, which consists of six to eight microtubular elements wound like a compressed spring (Fig. 4 and5). Twenty-two subpellicular microtubules originate from polar ring 2 and run longitudinally almost the entire length of the cell just beneath the inner membrane complex. In addition, two inner microtubules terminate in the conoid (Fig. 5). The microtubules are like a rib cage and are arranged in a gentle spiral. Individual microtubules have prominent transverse striations (121). Between the anterior tip and the nucleus, there are 8 to 10 club-shaped organelles (164) called rhoptries (Fig. 3and 6 to10). Rhoptries are excretory structures, each consisting of an anterior narrow neck up to 2.5 μm long that extends into the interior of the conoid, and a saclike, often labyrinthine posterior end (up to 1 μm long). Micronemes are rod-like structures which occur mostly at the anterior end of the parasite (Fig. 3 and 6).
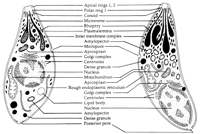

View larger version:
FIG. 3.
Schematic drawings of a tachyzoite (left) and a bradyzoite (right) of T. gondii. The drawings are composites of electron micrographs.
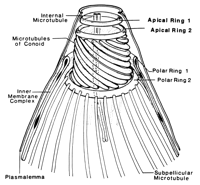

View larger version:
FIG. 4.
Schematic representation of the apical complex ofT. gondii. Modified from reference 51with permission of the publisher.


View larger version:
FIG. 5.
Transmission electron micrograph of a negatively stained apical complex of a tachyzoite. Ar1 and Ar2, apical rings 1 and 2; Co, conoid consisting of coiled microtubules; Im, internal microtubules; Pr 1, ring 1; Pr2, polar ring 2; Sm, subpellicular microtubules. Reprinted from reference 121 with permission of the publisher.


View larger version:
· In this page
· In a new window
· Download as PowerPoint Slide
FIG. 6.
Transmission electron micrograph of a tachyzoite of the VEG strain of T. gondii in a mouse peritoneal exudate cell. Am, amylopectin granule; Co, conoid; Dg, electron-dense granule; Go, Golgi complex; Mn, microneme; No, nucleolus, Nu, nucleus; Pv, parasitophorous vacuole; Rh, rhoptry.


View larger version:
FIG. 7.
Transmission electron micrograph of a tachyzoite of the VEG strain of T. gondiiundergoing endodyogeny within a mouse peritoneal macrophage to form two daughter tachyzoites. Cd, conoid of developing tachyzoite; Co, conoid of mother tachyzoite; Dg, electron-dense granule; Ga, Golgi adjunct; Hm, host cell mitochondrion; Hn, host cell nucleus; Id, inner membrane complex of developing tachyzoite; Im, inner membrane complex of mother tachyzoite; Mi, microchdrion; Mn, microneme; Nu, nuclei of daughter tachyzoites; Pl, plasmalemma of mother tachyzoite; Pm, parasitophorous vacuolar membrane; Pv, parasitophorous vacuole; Rh, rhoptries of daughter tachyzoites.


View larger version:
FIG. 8.
Transmission electron micrograph of four tachyzoites of the VEG strain of T. gondiiin the final stages of endodyogeny that are still attached by their posterior ends to a common residual body (Rb); note that several host cell mitochondria (∗) are situated close to the parasitophorous vacuole (Pv), which contains extensively developed tubulovesicular membranes (Tv). Am, amylopectin granule; Co, conoid; Dg, electron-dense granule; Hn, host cell nucleus; Mn, microneme; Mp, micropore; Nu, nucleus; Rh, rhoptry.


View larger version:
FIG. 9.
Transmission electron micrograph of a mouse peritoneal macrophage containing several tachyzoites of the VEG strain of T. gondii, one of which is escaping from the macrophage near the top of the micrograph (arrow). Note that the PV is no longer evident and the tachyzoites are free in the macrophage cytoplasm.
Although tachyzoites can move by gliding, flexing, undulating, and rotating, they have no visible means of locomotion such as cilia, flagella, or pseudopodia. The functions of the conoid, rhoptries, micropores, and micronemes are not fully known but are probably associated with host cell penetration and creation of an intracellular environment suitable for parasite growth and development. The conoid can rotate, tilt, extend, and retract as the parasite probes the host cell plasmalemma immediately before penetration (19). Rhoptries have a secretory function associated with host cell penetration, secreting their contents through the plasmalemma just above the conoid to the exterior (123). They contain a proteolytic enzyme (140a). The micropore is a cytosome-like structure formed by the invagination of the outer membrane of the pellicle (19, 123, 124).
Tachyzoites enter host cells (Fig. 10) by actively penetrating through the host cell plasmalemma or by phagocytosis (15, 54, 95, 120, 123, 125, 147, 152,160,182). After entering the host cell, the tachyzoite becomes ovoid and is surrounded by a parasitophorous vacuole (PV), which appears to be derived from both the parasite and the host cell. Soon after penetration, a tubulovesicular membranous network (TMN) develops within the PV (Fig. 8). Some of the TMN membranes are connected to the parasitophorous vacuolar membrane (145,149-151). The TMN appears to be derived from the posterior end of the tachyzoite (150). However, convoluted tubules, structurally similar to the TMN, were observed at the end of tachyzoites by Nichols et al. (123), and we have also observed similar structures in in vivo-cultured tachyzoites (Fig. 10).


View larger version:
FIG. 10.
Transmission electron micrograph of a tachyzoite of the VEG strain of T. gondiipenetrating a neutrophil in mouse peritoneum; note the moving junction (Mj) at the site of penetration into the neutrophil and the extraordinary early development of the tubulovesicular membranes (Tv) in the space between the neutrophil and the tip of the tachyzoite. Am, amylopectin granule; Dg, electron-dense granule; Rh, rhoptry; Nu, nucleus.
Tachyzoites multiply asexually within the host cell by repeated endodyogeny (Fig.7 and 8), a specialized form of reproduction in which two progeny form within the parent parasite (Fig. 3), consuming it (145). In endodyogeny, the Golgi complex divides first, becoming two complexes at the anterior end of the nucleus. Next, the anterior portions of the inner membrane complexes and the subpellicular microtubules of the progeny cells appear as two dome-shaped structures anteriorly. The parasite nucleus becomes horseshoe shaped, and the ends of the nucleus move into the dome-shaped anterior ends of the developing progeny. The inner membrane complex and subpellicular microtubules continue to extend posteriorly and surround one half of the nucleus, which eventually pinches into two. The progeny continue to grow until they reach the surface of the parent. The inner membrane complex of the parent disappears, and its outer membrane becomes the plasmalemma of the progeny cells. Tachyzoites continue to divide by endodyogeny (145). In vivo, most groups of tachyzoites are arranged randomly due to asynchronous cycles of endodyogeny. However, rosettes are occasionally formed due to synchronous division. In rapidly dividing tissue culture-adapted strains, T. gondiiwithin a vacuole may divide synchronously (14, 140), but this is not the norm. Rarely, tachyzoites of certain strains divide by binary fission (63,139). The host cell ruptures when it can no longer support the growth of tachyzoites (Fig. 8).
The rates of invasion and growth vary depending on the strain ofT. gondii and the type of host cells (1, 101). After entry of tachyzoites into a host cell, there is a variable lag period before the parasite divides, and this lag phase is partly parasite dependent (1). Mouse virulent strains of T. gondii grow faster in cell culture than do “avirulent” strains, and some strains of T. gondii form more rosettes than others (1). Although T. gondii isolates have been classified genetically into types I, II, and III (90, 94,148), there are no appreciable structural differences among different isolates of T. gondii.
Bradyzoites and Tissue CystsThe term “bradyzoite” (brady = slow in Greek) was also coined by Frenkel (73) to describe the organism multiplying slowly within a tissue cyst. Bradyzoites are also called cystozoites.
Tissue cysts (Fig. 11) grow and remain intracellular (Fig. 11C) as the bradyzoites divide by endodyogeny (64, 65). Tissue cysts vary in size; young tissue cysts may be as small as 5 μm in diameter and contain only two bradyzoites (Fig. 11B), while older ones may contain hundreds of organisms (Fig.11E). Tissue cysts in the brain are often spheroidal and rarely reach a diameter of 70 μm, whereas intramuscular cysts are elongated and may be 100 μm long (27, 36). Although tissue cysts may develop in visceral organs, including the lungs, liver, and kidneys, they are more prevalent in the neural and muscular tissues, including the brain, eyes, and skeletal and cardiac muscles (35). Intact tissue cysts probably do not cause any harm and can persist for the life of the host without causing a host inflammatory response.


View larger version:
FIG. 11.
Tissue cysts of T. gondii in mouse brains. (A) Tissue cyst with three bradyzoites, each with a terminal nucleus (arrows). Note the thin cyst wall (arrowhead). Impression smear with silver impregnation and Giemsa stain. (B) Three tissue cysts with well-defined cyst walls (arrowheads). Note the tissue cyst with two bradyzoites, each with a terminal nucleus (arrows). Impression smear with silver impregnation and Giemsa stain. (C) Intracellular tissue cyst in section. Note the thin cyst wall (arrow) and the host cell nucleus (arrowhead). Hematoxylin and eosin stain. (D) Tissue cyst with numerous PAS-positive bradyzoites (arrowheads) enclosed in a PAS-negative cyst wall (arrow). PASH stain. (E) Tissue cyst freed from mouse brain. Note the cyst wall (arrow) enclosing hundreds of bradyzoites. Unstained impression smear.
The tissue cyst wall is elastic and thin (<0.5 μm thick) (Fig. 11), and it encloses hundreds of crescent-shaped bradyzoites (Fig.12 and13), each approximately 7 by 1.5 μm in size (118). The tissue cyst develops within the host cell cytoplasm. The cyst wall is argyrophilic (77, 153), but results vary depending on the silver impregnation method used. According to Sims et al. (153), the cyst wall stains intensely with Bodian protoargol and Palmgren silver but not with methenamine silver. The lack of staining with methenamine silver indicates that the cyst wall contains no glycogen or other polysaccharides. The cyst wall is composed of host cell and parasite materials (64, 65, 153). It is ultimately lined with granular material, which also fills the space between the bradyzoites. Some bradyzoites degenerate (Fig. 13), especially in older tissue cysts (129). The cyst wall is only faintly periodic acid-Schiff (PAS) positive (Fig. 11D).


View larger version:
FIG. 12.
Transmission electron micrograph of two T. gondiitissue cysts in the brain of a mouse 6 months after infection with the Me-49 strain. Note that the tissue cyst on the left is younger than the one on the right because of the differences in their bradyzoites. The bradyzoites in the tissue cyst on the right contain more micronemes and amylopectin than do those in the one on the left. Additionally, the contents of rhoptries in the bradyzoites in the older tissue cyst are electron dense (arrowheads) whereas those in the bradyzoites in the younger tissue cyst are honeycombed (arrows). The cyst wall (cw) also is more branched and prominent in the older tissue cyst than in the younger tissue cyst. Reprinted from reference46.


View larger version:
FIG. 13.
Tissue cyst in the brain of a mouse that was inoculated 8 months earlier with oocysts of the VEG strain of T. gondii. This ultrathin section of the cyst shows approximately 110 bradyzoites (Bz). The tissue cyst is surrounded by a relatively thin cyst wall (Cw) and is situated within the host cell cytoplasm near the host cell nucleus (Hn). A few of the bradyzoites appear to be degenerate (Dz). The area within the rectangular box is shown at a higher magnification in Fig.14.
Bradyzoites (Fig. 3 and 12 to16) differ structurally only slightly from tachyzoites. They have a nucleus situated toward the posterior end, whereas the nucleus in tachyzoites is more centrally located. The contents of rhoptries in bradyzoites are usually electron dense, whereas those in tachyzoites are labyrinthine. However, the contents of rhoptries in bradyzoites vary with the age of the tissue cyst. Bradyzoites in younger tissue cysts may have labyrinthine rhoptries, whereas those in older tissue cysts are electron dense (Fig. 12). Also, most bradyzoites have one to three rhoptries, which are looped back on themselves (Fig. 3). Bradyzoites contain several amylopectin granules which stain red with PAS reagent; such material is either in discrete particles or absent in tachyzoites (Fig.3). Bradyzoites are more slender than are tachyzoites. Bradyzoites are less susceptible to destruction by proteolytic enzymes than are tachyzoites (93), and the prepatent period in cats following feeding of bradyzoites is shorter than that following feeding of tachyzoites (48).


View larger version:
FIG. 14.
High magnification of a portion of Fig. 13 showing the cyst wall and part of a bradyzoite with an active micropore. The cyst wall is approximately 0.25 to 0.75 μm thick and consists of a parasitophorous vacuolar membrane (Pm) that has numerous indentations (arrows); the rest of the wall consists of membrane-bound vesicles and moderately course granular material. Note that the micropore of the bradyzoite consists of an indentation in the parasite plasmalemma (Pl) and of an electron-dense collar (Ec) that arises from the inner membrane complex (Im); also note the presence of an electron-dense layer (EI) of material on the surface of the plasmalemma within the micropore. Am, amylopectin granule; Mn, microneme; Sm, subpellicular microtubule.


View larger version:
FIG. 15.
Bradyzoite in the tissue cyst shown in Fig. 13. Am, amylopectin granule; Ce, centrioles; Co, conoid; Dg, electron-dense granule; Ga, Golgi adjunct (apicoplast); Go, Golgi complex; Im, inner membrane complex; Mi, mitochondrion; Mn, microneme; Nu nucleus; Pm, plasmalemma; Rh, rhoptry.


View larger version:
FIG. 16.
Anterior end of a bradyzoite in Fig. 13 showing the apical complex. Am, amylopectin; Ar, apical rings 1 and 2; Co, conoid; Im, inner membrane complex; Mn, microneme; Nr, neck of rhoptry; Pl, plasmalemma; Pr, polar rings 1 and 2; Rh, rhoptry; Sm, subpellicular microtubule.
Enteroepithelial StagesCats shed oocysts after ingesting any of the three infectious stages of T. gondii, i.e., tachyzoites, bradyzoites, and sporozoites. Prepatent periods (time to the shedding of oocysts after initial infection) and frequency of oocyst shedding vary according to the stage of T. gondii ingested (37, 48, 80). Prepatent periods are 3 to 10 days after ingesting tissue cysts, ≥18 days after ingesting oocysts (37), and ≥13 days after ingesting tachyzoites (44). Fewer than 30% of cats shed oocysts after ingesting tachyzoites or oocysts, whereas nearly all cats shed oocysts after ingesting tissue cysts (37, 48).
After the ingestion of tissue cysts by cats, the cyst wall is dissolved by proteolytic enzymes in the stomach and small intestine. The released bradyzoites penetrate the epithelial cells of the small intestine and initiate the development of numerous generations of T. gondii (Fig. 17 and18). Five morphologically distinct types of T. gondii develop in intestinal epithelial cells before gametogony begins (47). These stages are designated types A to E instead of generations, because there are several generations within each T. gondii type (Fig. 17). Little has been added to the structure or biology of types A to C since the original description by Dubey and Frenkel (47).


View larger version:
FIG. 17.
Coccidian cycle of T. gondii. Reprinted from reference 47with permission of the publisher.


View larger version:
FIG. 18.
Enteroepithelial stages of T. gondii in a small intestinal villus of a cat fed tissue cysts. Note the heavy parasitization of entrocytes, containing schizonts (type D) (small arrows) and male (large arrow) and female (arrowheads) gamonts. Hematoxylin and eosin stain.
So far, only late stages (presumably type D) have been studied by transmission electron microscopy (TEM). These forms multiply by a specialized form of schizogony (Fig.19). As in normal schizogony, the nucleus divides two or more times without cytoplasmic division (61, 133, 144). Whether daughter organism (merozoite) formation begins after four or more nuclei have been formed is uncertain. Before or simultaneous with the last nuclear division, merozoite formation is initiated near the center of the schizont by the development of dome-shaped merozoite anlagen; whether one or two anlagen are formed near each nucleus is uncertain (61, 144). The merozoites eventually move towards the periphery of the schizont, and the schizont plasmalemma invaginates around each merozoite, forming the plasmalemma of the merozoite. The merozoites separate from the schizont at their posterior ends, sometimes leaving a residual body (47).
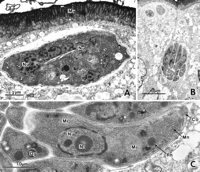

View larger version:
FIG. 19.
Transmission electron micrograph of type D T. gondiischizonts. (A) Intermediate schizont showing several nuclei (Nu). Mv, microvilli of host enterocyte. (B) Mature schizont. (C) Longitudinal section of a merozoite. Co, conoid; Dg, dense granule; Mi, mitochondrion; Mn, microneme; No, nucleolus; Nu, nucleus; Rh, rhoptry.
The schizogony observed in T. gondii type D organisms differs from schizogony in conventional coccidia (Eimeriaspecies) in that in T. gondii merozoites are formed internally and immature merozoites do not protrude from the schizont surface. It should be pointed out that many details of schizogony ofT. gondii are not clear even in type D organisms; nothing is known of the ultrastructures of T. gondii types to A to C described by Dubey and Frenkel (47).
Piekarski et al. (133) proposed the term “endopolygeny” to describe the division of T. gondii schizonts in feline enterocytes. They believed that merozoite formation began after two nuclear divisions. Vivier (175) had earlier used the term “endopolygeny” to describe observed divisional formation of more than two daughter T. gondii tachyzoites in the peritoneum of mice. We would like to propose that the term “endopolygeny” should not be used to describe schizogony in T. gondii but should be restricted to the merozoite formation in SarcocystisandFrenkelia spp. (51). In Sarcocystisendopolygeny, the nucleus becomes multilobed but does not divide into separate nuclei. Each nuclear lobe is eventually incorporated into developing merozoites. The number of merozoites inSarcocystis schizonts varies from 4 to 100 or more (51).
After the asexual development (types A to E), the sexual cycle starts 2 days after tissue cysts were ingested by the cat. The origin of gamonts has not been determined, but the merozoites released from schizont types D and E probably initiate gamete formation. Gamonts are found throughout the small intestine, but most commonly in the ileum, 3 to 15 days after inoculation (Fig. 18). They are found above the nucleus of the host epithelial cell near the tips of the villi of the small intestine (Fig. 20). Female gamonts are subspherical, and each contains a single centrally located nucleus and several PAS-positive granules. Ultrastructurally, the mature female gamete contains several micropores, rough and smooth endoplasmic reticulum, numerous mitochondria, double-membraned vesicles, and wall-forming bodies (WFB) (Fig. 20B). The double-membraned bodies are located near the nucleus and are probably derived from it (67). WFB are of two types: type I and type II. Type I WFB are about 0.35 μm in diameter and electron dense, and they appear before type II WFB (67). Type II WFB are moderately electron dense, less abundant than WFB, and larger than WFB (1.2 μm in diameter) (67).


View larger version:
FIG. 20.
Transmission electron micrograph of T. gondiigamonts. (A) Mature microgamont. Fl, flagellum of microgamete; Mg, body of microgamete; Pv, parasitophorous vacuole. (B) Zygote in the early stage of oocyst wall formation. Am, amylopectin granule; Lb, lipid body; Nu, nucleus of zygote; Ow, oocyst wall in the early stage of formation; Wf, wall-forming bodies.
Mature male gamonts (microgamonts) are ovoid to ellipsoidal in shape (Fig. 20A). During microgametogenesis, the nucleus of the microgamont divides to produce 10 to 21 nuclei (47). The nuclei move toward the periphery of the parasite and enter protuberances formed in the pellicle of the microgamont. One or two residual bodies remain in the microgamont after division into microgametes (Fig.20A). Microgametes are elongated and consist mainly of nuclear material. The anterior end is a pointed structure called the perforatorium, within which lie two basal bodies. Two long, free flagella originate from the basal bodies and project posteriorly. A large mitochondrion is situated near the basal bodies and just anterior to the nucleus. Five microtubules originate near the nucleus and extend posteriorly alongside it for a short distance.
Microgamonts have up to 21 microgametes. Microgametes use their flagella to swim to and penetrate and fertilize mature macrogametes to form zygotes. After fertilization, an oocyst wall is formed around the parasite (Fig. 20B). Infected epithelial cells rupture and discharge oocysts into the intestinal lumen. Five layers of the oocyst wall are formed at the surface of the zygote (67). No extensive cytoplasmic changes occur in the zygote while layers 1, 2, and 3 are formed, but type I WFBs disappear with the formation of layer 4 and type II WFBs disappear with the formation of layer 5.
OocystsUnsporulated oocysts are subspherical to spherical and are 10 by 12 μm in diameter (Fig. 21A). Under light microscopy, the oocyst wall consists of two colorless layers. Polar granules are absent, and the sporont almost fills the oocyst. Sporulation occurs outside the cat within 1 to 5 days of excretion depending upon aeration and temperature.

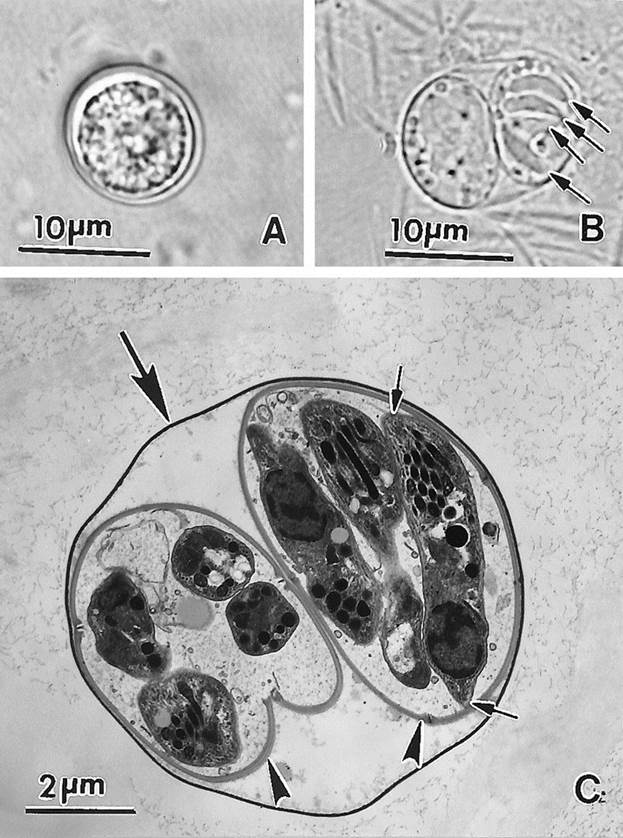
View larger version:
FIG. 21.
Oocysts of T. gondii. (A) Unsporulated oocyst. Note the central mass (sporont) occupying most of the oocyst. (B) Sporulated oocyst with two sporocysts. Four sporozoites (arrows) are visible in one of the sporocysts. (C) Transmission electron micrograph of a sporulated oocyst. Note the thin oocyst wall (large arrow), two sporocysts (arrowheads), and sporozoites, one of which is cut longitudinally (small arrows).
Sporulated oocysts are subspherical to ellipsoidal and are 11 by 13 μm in diameter (Fig. 21B and C). Each oocyst contains two ellipsoidal sporocysts without Stieda bodies. Sporocysts measure 6 by 8 μm. A sporocyst residuum is present; there is no oocyst residuum. Each sporocyst contains four sporozoites (Fig. 21C).
The ultrastructure of sporulation was described by Ferguson et al. (56-60). The cytoplasm of the unsporulated oocyst (zygote) has a large nucleus with amorphous nucleoplasm and a distinct nucleolus. The zygote is limited by a unit membrane with few micropores. The nucleus divides twice, giving rise to four nuclei, which are situated at the periphery of the zygote; at this stage a second limiting membrane is formed. After the cytoplasm divides, two spherical sporoblasts are formed, each with two nuclei (57).
As the sporulation continues, the sporoblasts elongate and sporocysts are formed. The two outer membranes of the sporoblasts become the outer layer of the sporocyst wall, and the plasmalemma of the cytoplasmic mass becomes the inner layer (58). Ultimately, as the sporocyst develops, four curved plates form the innermost layer of the sporocyst. The plates are joined by four liplike sutures (described below) with a depression on the surface of the sporocyst wall at the junction point. Sporozoite formation begins when two dense plaques (anlagen) appear at both ends of the sporocyst. Each nucleus divides into two and is incorporated into elongating sporozoite anlagen. Thus, four sporozoites are formed in each sporocyst (59). A prominent residual body is left after sporozoite are formed; the residual body is enclosed in a single-unit membrane (59).
Ultrastructurally, the oocyst wall of sporulated oocysts (Fig.22) consists of three layers: an electron-dense outer layer, an electron-lucent middle layer, and a moderately electron-dense inner layer (159). The middle layer consists of remnants of two membranes that evidently were laid down between the inner and outer layers during oocyst wall formation. Treatment of oocysts with 1.3% sodium hypochlorite (Clorox) removes the outer layer. The oocyst wall contains a single micropyle, which is relatively small and is located randomly in the oocyst wall (Fig.23). The micropyle is a 350-nm-diameter indentation that consists of three layers that are continuous with the three layers in the oocyst wall. However, the outer layer of the micropyle is thin and moderately electron dense and the inner layer is electron dense, disk shaped, and slightly thicker than the inner layer of the oocyst wall. Although the function of the micropyle is not known, it might represent a permeable site in the oocyst wall that is susceptible to the actions of CO2 and various enzymes that allow the entry of bile salts and trypsin, which stimulate the excystation of sporozoites from sporocysts.


View larger version:
FIG. 22.
Transmission electron micrograph of an oocyst of the VEG strain of T. gondiiexposed to excysting fluid (trypsin and bile salts) showing one sporocyst in an early stage of excystation. The oocyst is surrounded by a fine reticulate veil (Ov) and an oocyst wall (Ow). The sporocyst wall (Sw) consists of a continuous thin outer layer and an inner layer composed of four curved plates joined at sutures (arrows) containing an interposed strip of electron-dense material. During excystation, the excysting fluid acts on the sutures, causing the plates of the inner layer to separate and curl inward (double arrow), releasing the sporozoites. Am, amylopectin; Co, conoid; Dg, electron-dense granule; Lb, lipid body; Mn, microneme; Rh, rhoptry.


View larger version:
FIG. 23.
Transmission electron micrograph of a sporulated oocyst of the VEG strain of T. gondii. (A) Oocyst in the late stage of excystation, showing several free sporozoites (Sz) and collapsed sporocyst walls (Sw). Box B (see panel B) shows a micropyle in the oocyst wall (Ow). (B) High magnification of portion of panel A showing details of the oocyst wall and the micropyle, the oocyst wall consists of an outer electron-dense layer (Ol) and an inner electron-lucent layer (Il), which are separated by an electron-lucent line (El). At the micropyle, the outer layer of the oocyst wall becomes electron lucent and extremely thin (arrow); the electron-lucent line runs continuously through the micropyle, and the inner layer of the oocyst wall becomes slightly thicker near the micropyle and is continuous with the micropyle, which consists primarily of a curved, moderately electron-dense disc (∗).
The sporocyst wall consists of two distinct layers with a thin, electron-dense outer layer and thicker, moderately electron-dense inner layer. The inner layer consists of four curved plates (20,58) (Fig. 21 and 22). At sites of apposition between two plates, there are two apposing liplike thickenings and an interposed strip in the inner layer (20, 159). During excystation in the presence of bile salts and trypsin, the sporocyst ruptures suddenly, evidently at the sites of apposition between plates, releasing the sporozoites. As the plates separate, they curl inward to form conical coils (Fig. 23 and 24). The sporocyst residuum consists of amylopectin granules and lipid bodies.


·
FIG. 24.
Freshly excysted sporozoite still within an oocyst. Am, amylopectin; Co, conoid; Dg, electron-dense granule; Go, Golgi complex; Im, inner membrane complex: Lb, lipid body; Mi, mitochondrion; Mn, microneme; Ow, oocyst wall; Pl, plasmalemma; Rh, rhoptry; Sm, subpellicular microtubule; Sw, sporocyst wall.
Ultrastructurally, the sporozoite is similar to the tachyzoite, except that there is an abundance of micronemes, rhoptries, and amylopectin granules in the former. Sporozoites are 2 by 6 to 8 μm in size with a subterminal nucleus (Fig. 24 and25). There are no crystalloid bodies or any refractile bodies in T. gondiisporozoites (Fig. 24 and 25).


View larger version:
FIG. 25.
Schematic drawing of a T. gondiisporozoite.
The ultrastructural differences of some of the organelles in tachyzoites, bradyzoites, and sporozoites of the VEG strain of T. gondii are compared in Tables 1 and2; the VEG strain was isolated from the blood of an AIDS patient and is mildly virulent to mice depending on the stage of the parasite inoculated (42,49).
View this table:
TABLE 1.
Relative numbers of organelles and inclusion bodies in sporozoites, tachyzoites, and bradyzoites of the VEG strain ofT. gondii as determined by TEM
| Stage | Mean no. (range) ofd: | ||||
| Rhoptries | Micronemes | Dense granules | Amylopectin | Lipid | |
| Sporozoitea | 5.9 (2–11)4 | 55 (40–78) | 9.4 (5–15) | 7.8 (3–13) | 1.25 (1–3) |
| Tachyzoiteb | 6.7 (2–11) | 25 (19–38) | 9.1 (5–17) | 2.4 (1–6) | 0.6 (0–2) |
| Bradyzoitec | 5.5 (2–8) | 75.5 (36–112) | 2.7 (1–5) | 21.8 (7–38) |
· ↵a Sporozoites were freshly excysted from 34-day-old oocysts.
· ↵b Tachyzoites were obtained from the peritoneum of an IFN-γ knockout mouse 8 days after inoculation of tissue cysts.
· ↵c Bradyzoites were from cysts in the brains of mice at 8 months after inoculation of oocysts.
· ↵d Numbers represent means that were obtained by counting all organelles or inclusion bodies in 20 longitudinal sections of each type of zoite; ranges are given in parentheses.
Relative numbers of organelles and inclusion bodies in sporozoites, tachyzoites, and bradyzoites of the VEG strain ofT. gondii as determined by TEM
View this table:
TABLE 2.
Relative sizes of inclusion bodies in sporozoites, tachyzoites, and bradyzoites of the VEG strain of T. gondii as determined by TEM
| Stagea | Mean size (nm) (range) in: | ||
| Dense granules | Amylopectin | Lipid | |
| Sporozoite | 208 (175–250) | 356 (200–460) | 388 (200–550) |
| Tachyzoite | 244 (133–334) | 201 (103–333) | 224 (150–400) |
| Bradyzoite | 181 (167–201) | 358 (192–603) |
· ↵a For the source of these infectious stages, see Table 1, footnotes ato c.
·
Ultrastructural Comparison of Tachyzoites, Bradyzoites, and SporozoitesSporozoites, tachyzoites, and bradyzoites of T. gondiiare similar ultrastructurally but differ in certain organelles and inclusion bodies (Tables 1 and 2). All three zoites have similar numbers of rhoptries but the rhoptries differ in appearance between the zoites. For example, the rhoptries of tachyzoites are uniformly labyrinthine; sporozoites usually contain both labrynthine and uniformly electron-dense rhoptries; and bradyzoites usually contain uniformly electron-dense rhoptries, some of which are folded back on themselves (Fig. 3 and 15). Tachyzoites have few micronemes, sporozoites have an intermediate number, and bradyzoites have many. Dense granules are more numerous in sporozoites and tachyzoites than in bradyzoites (Fig. 3 and 25). Amylopectin granules are numerous and relatively large in sporozoites and bradyzoites but are few and small or absent in tachyzoites. Lipid bodies are numerous in sporozoites, rare in tachyzoites, and absent in bradyzoites (Fig. 3 and 25).
Attachment and Development of T. gondiiIn general, the attachment and penetration of host cells byT. gondii zoites (tachyzoites, bradyzoites, sporozoites, and merozoites) appear similar to those described for other coccidian parasites. The mechanical events involved in zoite attachment and penetration include (i) gliding of the zoite, (ii) probing of the host cell with the conoidal tip of the zoite, (iii) indenting the host cell plasmalemma, (iv) forming a moving junction that moves posteriorly along the zoite as it penetrates into the host cell, and (v) partially exocytosing micronemes, rhoptries, and dense granules. Zoites ofT. gondii can penetrate a variety of cell types from a wide range of hosts, indicating that the biochemical receptors involved in attachment and penetration are probably common to most animal cells. Host cell receptors consisting of laminin, lectin, and SAG1 are involved in T. gondiitachyzoite attachment and penetration (100). T. gondii zoites can, however, enter cells by means other than receptor-mediated penetration. Speer et al. (160) found that after passing completely through cells, some sporozoites of T. gondiicarried an envelope of host cell membranes and cytoplasm but were still capable of penetrating other cells. Zoites of T. gondii can also enter cells by being endocytosed (160).
Recently, a great deal of research on the formation of the PV has been conducted (96). As tachyzoites penetrate host cells, they are surrounded by a membrane that is evidently derived from the host cell plasmalemma minus host proteins. This membrane is destined to become the PV membrane (PVM), and a number of parasite proteins associate with it, including rhoptry proteins ROP2, ROP3, ROP4, and ROP7. ROP2 is located on the host cell cytoplasmic side of the PVM, suggesting that it plays a role in host-parasite biochemical communication (2). Within a few minutes after penetration, tachyzoites modify the newly formed PV and the PVM with parasite proteins and a TMN forms within the PV. The PVM acquires pore structures that freely allow charged molecules up to 1,200 kDa to diffuse bidirectionally between the PV and the host cell cytoplasm (142). Dense-granule proteins (GRA) are secreted into the PV after tachyzoite penetration, with GRA3 and GRA5 localizing on the PVM and GRA1, GRA2, GRA4, and GRA6 associating with the TMN. Collectively, these modifications establish a parasite-friendly environment within the host cell cytoplasm that is conducive to parasite replication.
To date, there is only a single report involving the expression of parasite antigens in T. gondii sporozoites. Speer et al. (161) found that GRA3 and SAG1 are developmentally regulated; they are not expressed in the sporozoite or the sporozoite-infected cell until 12 to 15 h after sporozoite inoculation of cell cultures. The first parasite multiplication occurs after the expression of GRA3 and SAG1, indicating that although the sporozoite is competent for cell penetration, it is not immediately able to establish an intracellular environment which can support replication. Therefore, many of the proteins necessary for parasite growth appear to be down-regulated in the sporozoite, perhaps due to dormancy within the oocyst.
Most of the available information concerning the interaction ofT. gondii zoites with host cells has been derived from in vitro cultivation with tachyzoites. There is limited information on in vivo zoite-host cell interactions. Recent studies with sporozoites have shown that T. gondii interacts with host cells substantially differently in vivo from the interaction in vitro. For example, Speer et al. (161) found that in cultured cells, T. gondii sporozoites induced the formation of two types of PVs. Type 1 PVs formed first, were relatively large (20 to 30 μm in diameter), had an indistinct PVM, and contained no exocytosed dense granule material or a TMN. After 12 to 18 h, sporozoites in type 1 PVs actively penetrated into the host cell cytoplasm and established type 2 PVs, which contained exocytosed dense granules, a TMN, and a distinct PVM. Parasites multiplied by endodyogeny in type 2 PVs but did not multiply in type 1 PVs. In follow-up studies in mice, it was found that sporozoites passed through enterocytes and goblet cells of the mouse intestinal epithelium and entered the lamina propria, where they infected all cells of the host except erythrocytes and underwent endodyogeny to form tachyzoites (52, 158). In contrast to the in vitro studies, sporozoites did not form type 1 PVs at any point while they were in the intestinal epithelium or the lamina propria. Even though the sporozoites were just passing through ileal enterocytes, they still formed PVs that contained exocytosed dense granule material and well-developed TMNs, which appear necessary for parasite multiplication (102, 106, 149, 150). The presence of exocytosed material and TMNs associated with the PVs of sporozoites in transit across the intestinal epithelium indicates that parasite replication does not always follow the formation of a parasite-modified PV. After entering cells in the lamina propria, PVs equivalent to the type 2 PVs were again formed, but here the sporozoites multiplied by endodyogeny. These findings therefore indicate that the type 1 PV seen in cultured cells might represent an anomaly of in vitro cultivation.
Bjerkås (5) and Sibley et al. (150) suggested that the TMN was formed in association with a transient sac-like structure at the posterior end of the tachyzoite. However, as stated above, TMN can develop early near the anterior of tachyzoites even before the parasite completely enters the host cell (Fig. 10).
Previous SectionNext Section
Date: 2016-01-03; view: 1181
| <== previous page | | | next page ==> |
| TEXT 5. THE POWER OF SELF-TALK | | | DEVELOPMENT AND BIOLOGY OF BRADYZOITES AND TISSUE CYSTS IN VIVO |