CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
BRADYZOITES AND TISSUE CYST FORMATION IN CELL CULTUREHistoryEven though Hogan et al. (89) were the first to report the presence of T. gondiitissue cysts in cell culture, little interest in in vitro cultivation of tissue cysts was shown over the next 25 to 30 years, and few reports appeared in the literature (88, 97, 98, 114, 146). Hoff et al. (88) demonstrated that in vitro-produced tissue cysts led to oocyst excretion in cats, indicating that the tissue cysts were biologically the same as those produced in vivo. A renewed interest in studying the biology of tissue cysts and bradyzoites was evidenced once the importance of toxoplasmic encephalitis in AIDS patients was recognized in the mid-1980s. Improved methods for the induction and detection of tissue cysts were developed in the early 1990s. Many researchers are now using in vitro systems to investigate many exciting aspects of bradyzoite-tachyzoite and tachyzoite-bradyzoite interconversion and the development of tissue cysts. Studies concerning the development of tissue cysts and conversion of tachyzoites to bradyzoites and of bradyzoites to tachyzoites are not always clear-cut because of the terminology used to describe stages and the gradualness of the conversion process (85). For example, is a PV that contains tachyzoites and bradyzoites, as indicated by immunostaining, a tissue cyst, and if TEM demonstrates a tissue cyst wall surrounding what are structurally tachyzoites, is it a tissue cyst? The results of observations in vitro should always be compared to what is already known about tissue cyst production in vivo (74). Evidence for Tissue Cyst FormationThe methods used to determine whether tissue cysts and bradyzoites are present in vitro are widely varied. Light microscopy.Light microscopy is probably the least definitive of all the methods used to demonstrate tissue cysts in vitro because it is impossible to distinguish bona fide tissue cysts from large groups of tachyzoites. It is somewhat easier to identify free-floating tissue cysts that originated from host cell rupture and are floating in the media in cell cultures, but confusion with groups of tachyzoites still cannot be ruled out. Phase-contrast microscopy may provide a more definitive identification of these stages because the tissue cyst wall is phase lucent (178, 179). Identification of tissue cysts in cultures stained with Giemsa, PAS, or silver stains can also be highly subjective; PAS and silver stains are superior to Giemsa. The age at which tissue cysts become PAS or silver positive has not been examined critically. Immunostaining with specific monoclonal or polyclonal antibodies is highly specific and is the technique currently used by most researchers (see below). Quantitative studies that use only unstained or histochemically stained cultures must be interpreted with caution. Acid-pepsin resistance.The tachyzoites of T. gondii are more susceptible to digestion by acid-pepsin solution than are the bradyzoites (93). Resistance to acid-pepsin digestion was used to determine the numbers of bradyzoites in a cell culture in a modified plaque assay (134). However, this method cannot consistently distinguish tachyzoites from bradyzoites (see below). Transmission electron microscopy.TEM can be used to demonstrate tissue cysts and the localization of antigens in bradyzoites and tissue cysts. The matrix of tissue cysts produced in vitro is often absent or greatly reduced (113, 116) compared to tissue cysts in mouse brain (65). Free-floating tissue cysts that originated from host cell rupture during processing for TEM are most likely to exhibit a lack of matrix material. Tissue cysts that form in vitro are generally smaller than those found in vivo, and the identity of the host cell is important (87). Young tissue cysts many contain odd numbers of organisms (Fig.30) (89,154, 179). Generally, it is difficult to obtain quantitative data by TEM because of the small amount of material used for examination. Immunoelectron microscopy has been used to determine the expression and localization of bradyzoite-specific antigens in vitro (85, 86, 172, 179).
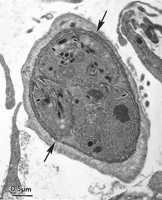
View larger version: FIG. 30. Transmission electron micrograph of a T. gondiitissue cyst in human foreskin fibroblast culture (HS68) 6 days after inoculation with the VEG strain zoites. Note the three bradyzoites with honeycombed rhoptries (arrowheads) and a thin cyst wall (large arrows). The small arrows point to the plasmalemma of the bradyzoites. Only the bradyzoite stage of T. gondii will reliably produce oocyst excretion in cats. The prepatent period is short in orally acquired bradyzoite-induced infections and can be used to determine if tissue cysts are present in cell cultures (88, 109). By using TEM and by bioassay in cats fed T. gondii, it was determined that tissue cysts were present on day 3 p.i. but were not infectious for cats until day 6 p.i. (109). Repeated passage of T. gondii in cell cultures results in the loss of the ability of bradyzoites to induce oocyst excretion in cats after about 40 passages in vitro (109), a phenomenon similar to that which occurs after repeated passage of tachyzoites in mice (74a). Functional bradyzoites of the oocystless T263 strain of T. gondii have been produced in cell culture and used to orally vaccinate cats against oocyst excretion (134). Immunostaining.With the identification of stage-specific antigens and the subsequent generation of bradyzoite and tissue cyst stage-specific monoclonal antibodies, methods for closely examining the developmental biology of T. gondii tissue cysts became available (9, 10, 13, 86, 99, 171, 178, 181,183). By using both tachyzoite- and bradyzoite-specific monoclonal antibodies, it was determined that tachyzoites and bradyzoites could both be present in the same PV (10, 155, 156,179), indicating that stage conversion from tachyzoite to bradyzoite is asynchronous. Immunostaining permits accurate quantification of specific stages and is important in studies designed to determine the ability of agents to induce tissue cyst formation in vitro. Host Cells and T. gondii StrainsThe types of host cells and T. gondii strains used to study tissue cyst and bradyzoite development in vitro have been widely varied (Table 4). The majority of studies have been done with fibroblast cell types (mainly of human origin) because these cells are easy to grow and usually survive for extended periods as an intact monolayer. Most cell lines will support tissue cyst development, and the type of cell line probably does not contribute greatly to the presence or absence of tissue cyst formation (109, 116). Most strains of T. gondii will produce tissue cysts in vitro (113, 116). Strains with low replication rates, which are less pathogenic for mice (i.e., VEG, ME-49, Beverley, Prugniaud, and NTE), usually produce more tissue cysts than do the more rapidly dividing, pathogenic strains (i.e., RH and BK) (83, 114). Before the use of methods to induce tachyzoite-to-bradyzoite conversion, strains of T. gondiithat were less pathogenic for mice produced tissue cysts with larger numbers of zoites (both bradyzoites and tachyzoites) than did highly pathogenic strains (11, 83). View this table: TABLE 4. Combinations of host cell types, T. gondiistrains, and infective stages that have been observed to produce bradyzoites and tissue cysts
· ↵a PM, primary; MP, macrophage. · ↵b MAb, monoclonal antibody; APD, acid-pepsin digestion; PAb, polyclonal antibody; LM, light microscopy; G, = Giemsa type stain; SS, silver stain. · ↵c B, bradyzoites; T, tachyzoites; S, sporozoites; Z, mixture of bradyzoites and tachyzoites; T?, listed as tachyzoites by authors but probably a mixture of zoites; UD, stage not determined. Methods of Tissue Cyst InductionMost strains of T. gondii will spontaneously develop tissue cysts in cell culture with no manipulation (22, 109, 111,113, 156). However, the number of tissue cysts produced spontaneously is small and manipulation of the cell culture system is needed to increase this number (11, 83, 155, 156, 178). Early on, the use of T. gondii antiserum in the culture medium was used to promote tissue cyst formation in vitro (88, 98,146). The results of these studies are difficult to interpret because questionable methods of identification of tissue cysts were used (98, 146) or tissue cyst numbers were not quantifiable (88). Popiel et al. (134) achieved an enhanced rate of tissue cyst formation in cultures treated with 5% rabbit anti-T. gondii serum but attributed the enhancement to a reduction in the replication of tachyzoites. It remains unclear if anti-T. gondiiserum actually induces bradyzoite and tissue cyst formation. Tachyzoite-to-bradyzoite conversion can be induced by applying external stress to various types of infected cell lines. Many investigators use pH manipulation to induce in vitro stage conversion. Both acidic (pH 6.6) and basic (pH 8.0 to 8.2) manipulations will lead to the conversion of tachyzoites to bradyzoites (155,179). Most researchers use the pH 8 treatment. Exposure of extracellular tachyzoites to medium of pH 8.1 for 1 h increases the formation of bradyzoites and tissue cysts (180). Temperature stress (40°C) and chemical stress (sodium arsenite) will also induce tachyzoite-to-bradyzoite transformation (157). However, sodium arsenite treatment did not result in the production of tissue cysts when examined by TEM (155). IFN-γ may act to inhibit tachyzoite replication in static cell cultures and permit the spontaneous development of tissue cysts (97). Although IFN-γ does not cause increased expression of bradyzoite-specific antigens or tissue cyst production in human fibroblasts (155), it is highly effective in inducing tissue cyst formation in cultured macrophages. IFN-γ treatment of murine macrophages induces the release of nitric oxide (NO), which reduces tachyzoite replication and induces the expression of bradyzoite-specific antigens (9, 11). If IFN-γ induction of NO release is inhibited by treatment with polymyxin or anti-tumor necrosis factor, no inhibition of tachyzoite replication and no increased expression of bradyzoite antigens occur. The highest level of bradyzoite antigen expression is found in macrophages where the NO release is manipulated to be 40 to 65% of maximum (11). Exogenous NO supplied by sodium nitroprusside will also inhibit tachyzoite replication and induce bradyzoite antigen expression in murine macrophages and in host cells with nonfunctional mitochondria (12). Released NO reacts with iron-sulfur centers of proteins and therefore reacts with several proteins involved in electron transport. The activity of NO in host cells lacking functional mitochondria indicates that its effect is exerted on parasite mitochondria and not on host cell mitochondria. Mitochondrial inhibitors (oligomycin, antimycin A, atovaquone, rotenone, myxothiazol, and carbonyl cyanidem-chlorophenylhydrazone), which affect different aspects of mitochondrial function, will also induce tachyzoite-to-bradyzoite stage conversion (11, 69, 170). Studies involving host cells with nonfunctional mitochondria indicate that the inhibitors are affecting the T. gondii mitochondrian and not the host cell mitochondria (11, 170). A common mode of action of the pH manipulations, sodium arsenite treatment, 43°C treatment, NO, and mitochondrial inhibitors is that they can be associated with the induction of heat shock proteins (HSP) (9, 180). The relationship of HSP to these stress inducers is being actively investigated in several laboratories. Bohne et al. (8) have cloned and characterized a bradyzoite-specifically expressed gene (hsp30/BAG-1), which is related to genes encoding small HSP of plants. BAG-1 is located in the cytoplasm of bradyzoites, and expression of the 30-kDa antigen appears to be regulated at the mRNA level (8). Weiss et al. (180) have shown that a monoclonal antibody to the inducible form of HSP70 reacts specifically with bradyzoites and recognizes a 72-kDa antigen. If quercetin is used to inhibit the synthesis of HSP, induction of bradyzoite antigen expression is also inhibited. The roles that HSPs play in bradyzoite development and induction will be an active area of research over the next several years. Current Knowledge of the Events of Tissue Cyst DevelopmentSporozoites, tachyzoites, and bradyzoites can develop into tissue cysts in vitro. If sporozoites are used as the inoculum, the appearance of tissue cysts seems to be delayed by several days (109). Ultrastructurally, tissue cysts have been observed as early as 2 days after inoculation of a mixture of tachyzoites and bradyzoites (111), and several TEM studies have documented tissue cysts by 3 days p.i. (109,179). Tissue cysts present by 3 days p.i. are not biologically mature, because they do not induce oocyst excretion in cats. Immunostaining studies of cultured cells have identified a small population of tachyzoites that express bradyzoite- and tachyzoite-specific antigens (10, 155), which most probably represent transitional stages. In the absence of any induction treatment, tachyzoites obtained from the peritoneal cavity of mice do not react with bradyzoite-specific antibodies, but by 24 h in culture, a small population will express both tachyzoite and bradyzoite antigens, and cells containing only bradyzoite-reactive organisms are present by 3 days (156). Maximal expression of bradyzoite-specific antigens occurs 4 days after inoculation of NTE tachyzoites in pH 8-induced cell cultures (85). When mouse brain-derived bradyzoites are used as the inoculum in the absence of any induction treatment, the bradyzoites begin to express tachyzoite-specific antigens as early as 15 h (156). Mixed populations were observed at 24 h, and cells containing only tachyzoites or only bradyzoites were observed at 48 h. The observation of groups of parasites that were reactive with only bradyzoite-specific antibodies indicates that the inoculated bradyzoites can produce additional bradyzoite-reactive stages. The source of tachyzoites and the strain of T. gondii may influence the development of bradyzoites. According to Appleford and Smith (1), a small population of organisms from peritoneal exudates of mice contain bradyzoites as revealed by staining with a bradyzoite-specific monoclonal antibody (Pb36). Methods for Tissue Cyst and Bradyzoite IsolationFew data are available on the isolation of T. gondiitissue cysts from cell cultures. Soête et al. (155) isolated pH 8- or 43°C-induced tissue cysts of the RH strain from Vero or human fibroblast cells by scraping the cells from the monolayer and rupturing host cells with a Dounce homogenizer. Low-speed centrifugation resulted in a sediment that contained tissue cysts and free zoites. Bohne et al. (9) used magnetic cell sorting to isolate pH 8-induced bradyzoites of the NTE strain of T. gondii grown in L929 fibroblasts. A surface-reactive bradyzoite-specific antibody was used to purify the bradyzoites from a mixture of bradyzoites and tachyzoites. The final product consisted of 95 to 98% pure bradyzoites free of host cell contaminants. Date: 2016-01-03; view: 840
|