
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Mechanism of biological nitrogen fixation
Biological nitrogen fixation can be represented by the following equation, in which two moles of ammonia are produced from one mole of nitrogen gas, at the expense of 16 moles of ATP and a supply of electrons and protons (hydrogen ions):
N2 + 8H+ + 8e- + 16 ATP = 2NH3 + H2 + 16ADP + 16 Pi
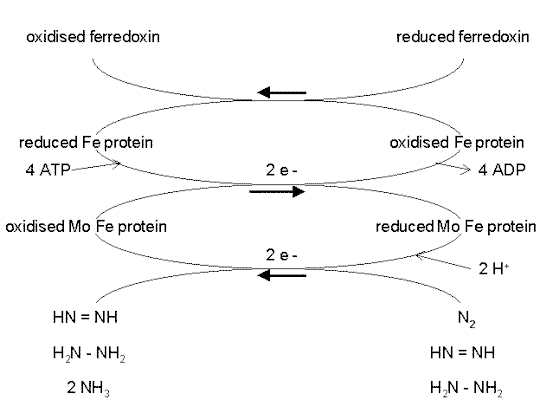
This reaction is performed exclusively by prokaryotes (the bacteria and related organisms), using an enzyme complex termed nitrogenase. This enzyme consists of two proteins - an iron protein and a molybdenum-iron protein, as shown below.
The reactions occur while N2 is bound to the nitrogenase enzyme complex. The Fe protein is first reduced by electrons donated by ferredoxin. Then the reduced Fe protein binds ATP and reduces the molybdenum-iron protein, which donates electrons to N2, producing HN=NH. In two further cycles of this process (each requiring electrons donated by ferredoxin) HN=NH is reduced to H2N-NH2, and this in turn is reduced to 2NH3.
Depending on the type of microorganism, the reduced ferredoxin which supplies electrons for this process is generated by photosynthesis, respiration or fermentation.
There is a remarkable degree of functional conservation between the nitrogenase proteins of all nitrogen-fixing bacteria. The Fe protein and the Mo-Fe protein have been isolated from many of these bacteria, and nitrogen fixation can be shown to occur in cell-free systems in a laboratory when the Fe protein of one species is mixed with the Mo-Fe protein of another bacterium, even if the species are very distantly related.
Date: 2015-01-29; view: 2591
| <== previous page | | | next page ==> |
| Role of nitrogen in the biosphere | | | The nitrogen-fixing organisms |