
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Covalent Bonding
The other three reactions shown above give products that are very different from sodium chloride. Water is a liquid at room temperature; carbon dioxide and carbon tetrafluoride are gases. None of these compounds is composed of ions. A different attractive interaction between atoms, called covalent bonding, is involved here. Covalent bonding occurs by a sharing of valence electrons, rather than an outright electron transfer. Similarities in physical properties (they are all gases) suggest that the diatomic elements H2, N2, O2, F2 & Cl2 also have covalent bonds.
Examples of covalent bonding shown below include hydrogen, fluorine, carbon dioxide and carbon tetrafluoride. These illustrations use a simple Bohr notation, with valence electrons designated by colored dots. Note that in the first case both hydrogen atoms achieve a helium-like pair of 1s-electrons by sharing. In the other examples carbon, oxygen and fluorine achieve neon-like valence octets by a similar sharing of electron pairs. Carbon dioxide is notable because it is a case in which two pairs of electrons (four in all) are shared by the same two atoms. This is an example of a double covalent bond.
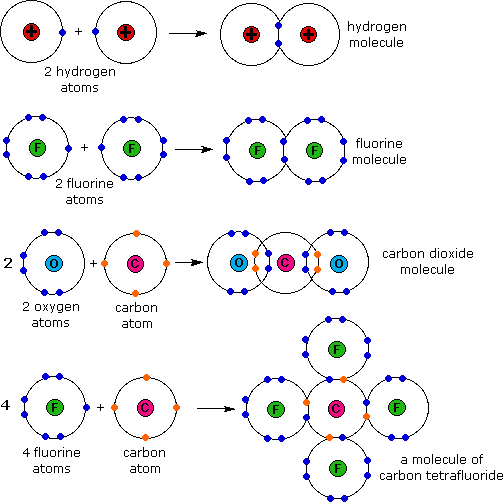
These electron sharing diagrams (Lewis formulas) are a useful first step in understanding covalent bonding, but it is quicker and easier to draw Couper-Kekulé
formulas in which each shared electron pair is represented by a line between the atom symbols. Non-bonding valence electrons are shown as dots. These formulas are derived from the graphic notations suggested by A. Couper and A. Kekulé, and are not identical to their original drawings. Some examples of such structural formulas are given in the following table.
|
Multiple bonding, the sharing of two or more electron pairs, is illustrated by ethylene and formaldehyde (each has a double bond), and acetylene and hydrogen cyanide (each with a triple bond). Boron compounds such as BH3 and BF3 are exceptional in that conventional covalent bonding does not expand the valence shell occupancy of boron to an octet. Consequently, these compounds have an affinity for electrons, and they exhibit exceptional reactivity when compared with the compounds shown above.
Valence
The number of valence shell electrons an atom must gain or lose to achieve a valence octet is called valence. In covalent compounds the number of bonds which are characteristically formed by a given atom is equal to that atom's valence. From the formulas written above, we arrive at the following general valence assignments:
| Atom | H | C | N | O | F | Cl | Br | I |
| Valence |
The valences noted here represent the most common form these elements assume in organic compounds. Many elements, such as chlorine, bromine and iodine, are known to exist in several valence states in different inorganic compounds.
| Charge Distribution |
Date: 2015-01-29; view: 1262
| <== previous page | | | next page ==> |
| Chemical Bonding and Valence | | | Charge Distribution |
