
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Chemistry FINAL
1.Classification of low-molecular compounds. Organic chemistry is a chemistrysubdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbonatoms.The objects of study in organic chemistry include hydrocarbons, compounds containing only carbon and hydrogen, as well as compositions based on carbon but containing other elements.Organic chemistry overlaps with many areas including medicinal chemistry, biochemistry, organometallic chemistry, and polymer chemistry, as well as many aspects of materials science. According to structure of skeleton carbohydrates can be divided into two: aliphatic (open) and cyclic (closed). Aliphatic, in its turn, is divided into two: saturated and unsaturated. Saturated – alkane CnH2n+2. Unsaturated – alkene CnH2n, alkadiene CnH2n-2, alkyne CnH2n-2.In organic chemistry, a saturated compound has no double or triple bonds or ring. In saturated hydrocarbons, every carbon atom is attached to two hydrogen atoms, except those at the ends of the chain, which bear three hydrogen atoms. In the case of saturated ethane, each carbon centre has four single bonds as is characteristic of other saturated hydrocarbons, alkanes. In contrast, in ethylene (C2H4), each carbon centre is engaged in two single and one double bond. Thus, like other alkenes, ethylene is unsaturated. The degree of unsaturation specifies the amount of hydrogen that a compound can bind. Unsaturated is used when any carbon structure contains double or occasionally triple bonds. Cyclic: carbocyclic (is divided into two: alicyclic and aromatic-benzene) and heterocyclic. According to the number of functional groups: monofunctional, polifunctional, heterofunctional.Functional group, in organic chemistry, a group of atoms responsible for the characteristic chemical reactions of a molecule.
2.Primary and secondary metabolites, characteristic, function in the organism.Metabolites are compounds synthesized by plants for both essential functions, such as growth and development (primary metabolites), and specific functions, such as pollinator attraction or defense against herbivory (secondary metabolites). Primary Metabolites.Primary metabolites are involved in growth, development, and reproduction of the organism. The primary metabolite is typically a key component in maintaining normal physiological processes; thus, it is often referred to as a central metabolite. Primary metabolites are typically formed during the growth phase as a result of energy metabolism, and are deemed essential for proper growth. Primary metabolites comprise many different types of organic compounds, including, but not limited to, carbohydrates, lipids, proteins, and nucleic acids. They are found universally in the plant kingdom because they are the components or products of fundamental metabolic pathways or cycles such as glycolysis, the Krebs cycle, and the Calvin cycle. Because of the importance of these and other primary pathways in enabling a plant to synthesize, assimilate, and degrade organic compounds, primary metabolites are essential. Examples of primary metabolites include alcohols such as ethanol, lactic acid, and certain aminoacids. Within the field of industrial microbiology, alcohol is one of the most common primary metabolites used for large-scale production. Specifically, alcohol is used for processes involving fermentation which produce products like beer and wine. Additionally, primary metabolites such as amino acids-- including L-glutamate and L-lysine, which are commonly used as supplements-- are isolated via the mass production of a specific bacterial species, Corynebacteriaglutamicum. Another example of a primary metabolite commonly used in industrial microbiology includes citric acid. Citric acid, produced by Aspergillusniger, is one of the most widely used ingredients in food production. It is commonly used in pharmaceutical and cosmetic industries as well. Secondary Metabolites.Secondary metabolites are typically organic compounds produced through the modification of primary metabolite synthases. Secondary metabolites largely fall into three classes of compounds: alkaloids, terpenoids, and phenolics. However, these classes of compounds also include primary metabolites, so whether a compound is a primary or secondary metabolite is a distinction based not only on its chemical structure but also on its function and distribution within the plant kingdom. Secondary metabolites do not play a role in growth, development, and reproduction like primary metabolites do, and are typically formed during the end or near the stationary phase of growth. Many of the identified secondary metabolites have a role in ecological function, including defense mechanism, by serving as antibiotics and by producing pigments. Examples of secondary metabolites with importance in industrial microbiology include atropine and antibiotics such as erythromycin and bacitracin. Atropine, derived from various plants, is a secondary metabolite with important use in the clinic. Atropine is a competitive antagonist for acetycholine receptors, specifically those of the muscarinic type, which can be used in the treatment of bradycardia.Antibiotics such as erythromcyin and bacitracin are also considered to be secondary metabolites. Erythromycin, derived from Saccharopolysporaerythraea, is a commonly used antibiotic with a wide antimicrobial spectrum. Another example of an antibiotic which is classified as a secondary metabolite is bacitracin. Bacitracin, derived from organisms classified under Bacillus subtilis, is an antibiotic commonly used a topical drug. Bacitracin is synthesized in nature as a nonribosomal peptide synthetase that can synthesize peptides; however, it is used in the clinic as an antibiotic. Primary and secondary metabolites are often used in industrial microbiology for the production of food, amino acids, and antibiotics.
3.Types of chemical bonds in low-molecular biological molecules. Characteristics, formation of chemical bonds, properties.
 Chemical bond – the interaction between the atoms, leading to the formation of molecules or crystals. In organic compounds there are two main types of chemical bond: Covalent and Ionic.Ionic bond. Found in organic compounds are rarely.
Chemical bond – the interaction between the atoms, leading to the formation of molecules or crystals. In organic compounds there are two main types of chemical bond: Covalent and Ionic.Ionic bond. Found in organic compounds are rarely.
Covalent bond is the main in organic compounds. Such a relationship is formed by socialization of electrons pairs between two atoms. Classification of covalent bonds I. By polarity  . II. On the symmetry of orbitals:s -bond - covalent bond formed by the overlapping atomic orbitals along the axis, which connecting the nucleus of atoms: p-bond – covalent bond formed by lateral overlapingnonhybridp-orbitals. The p-atomic orbitals delocalized, forming a p-orbitals: d-bond – bond formed by frontal d-orbitals overlap:
. II. On the symmetry of orbitals:s -bond - covalent bond formed by the overlapping atomic orbitals along the axis, which connecting the nucleus of atoms: p-bond – covalent bond formed by lateral overlapingnonhybridp-orbitals. The p-atomic orbitals delocalized, forming a p-orbitals: d-bond – bond formed by frontal d-orbitals overlap:  III. Classification of covalent bonds by forming way.The exchange mechanism are involved one electron atomic orbitals. Every atom gives an electron to form common 1 pair:Donor-acceptor mechanism – bond formed by the electron pair of donor and vacant (free) acceptor orbitals. IV Classification of covalent bonds by order of bonds:1. single (one s -bond) for example between carbon in the molecule ethane.2. double (s -1 and 1 p -bonds), for example, in a molecule of ethylene.3. triple (1 s -link and 2 p), for example, in a molecule of acetylene.4. quarter (1 s -link, 2 p- and 1 d -link) – found in inorganic compounds between atoms of metals such as between the two atoms of metals (Cr, Mo) the formation of five and even six bonds.Characteristics of covalent bonds:Ι. Energy.II. Length of bond.III.Polarity.IV. ThePolarizability. Hydrogen bond – the attraction of protonized hydrogen atoms, attached to theelectonegative atom or any other atom with a negative charge.
III. Classification of covalent bonds by forming way.The exchange mechanism are involved one electron atomic orbitals. Every atom gives an electron to form common 1 pair:Donor-acceptor mechanism – bond formed by the electron pair of donor and vacant (free) acceptor orbitals. IV Classification of covalent bonds by order of bonds:1. single (one s -bond) for example between carbon in the molecule ethane.2. double (s -1 and 1 p -bonds), for example, in a molecule of ethylene.3. triple (1 s -link and 2 p), for example, in a molecule of acetylene.4. quarter (1 s -link, 2 p- and 1 d -link) – found in inorganic compounds between atoms of metals such as between the two atoms of metals (Cr, Mo) the formation of five and even six bonds.Characteristics of covalent bonds:Ι. Energy.II. Length of bond.III.Polarity.IV. ThePolarizability. Hydrogen bond – the attraction of protonized hydrogen atoms, attached to theelectonegative atom or any other atom with a negative charge.
4.Covalent bond, characteristics. Types of covalent bond.A covalent bond is the chemical bond that involves the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Covalent bonding includes many kinds of interaction, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, and three-center two-electron bonds. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding.[5]Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require the two atoms be of the same elements, only that they be of comparable electronegativity. Although covalent bonding entails sharing of electrons, it is not necessarily delocalized. Physical properties of covalent compounds (polar and non-polar). Covalent bonds are affected by the electronegativity of the connected atoms. Two atoms with equal electronegativity will make nonpolar covalent bonds such as H−H. An unequal relationship creates a polar covalent bond such as with H−Cl. There are three types of covalent bond depending upon the number of shared electron pairs. A covalent bond formed by the mutual sharing of one electron pair between two atoms is called a "Single Covalent bond."It is denoted by single short line.
 A covalent bond formed between two atoms by the mutual sharing of two electron pairs is called a "double covalent bond". It is denoted by double short line.
A covalent bond formed between two atoms by the mutual sharing of two electron pairs is called a "double covalent bond". It is denoted by double short line. 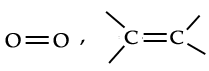 . A covalent bond formed by the mutual sharing of three electron pairs is called a "Triple covalent bond". It is denoted by triple short line.
. A covalent bond formed by the mutual sharing of three electron pairs is called a "Triple covalent bond". It is denoted by triple short line. 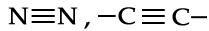 .A covalent bond formed between two different atoms is known as Polar covalent bond. For example when a Covalent bond is formed between H and Cl , it is polar in nature because Cl is more electronegative than H atom . Therefore, electron cloud is shifted towards Cl atom. Due to this reason a partial -ve charge appeared on Cl atom and an equal +ve charge on H atom.
.A covalent bond formed between two different atoms is known as Polar covalent bond. For example when a Covalent bond is formed between H and Cl , it is polar in nature because Cl is more electronegative than H atom . Therefore, electron cloud is shifted towards Cl atom. Due to this reason a partial -ve charge appeared on Cl atom and an equal +ve charge on H atom.  . A covalent bond formed between two like atoms is known as Non-polar bond. Since difference of
. A covalent bond formed between two like atoms is known as Non-polar bond. Since difference of
electro negativity is zero therefore, both atoms attract electron pair equally and no charge appears on any atom and the whole molecule becomes neutral.H – H.Cl-Cl.F – F.
5. The effects of atoms in molecules. Inductive and mesomeric effects.The mesomeric effect or resonance effect in chemistry is a property of substituents or functional groups in a chemical compound. The effect is used in a qualitative way and describes the electron withdrawing or releasing properties of substituents based on relevant resonance structures and is symbolized by the letter M. The mesomeric effect is negative (-M) when the substituent is an electron-withdrawing group and the effect is positive (+M) when based on resonance and the substituent is an electron releasing group. Examples of -M substituents: acetyl - nitrile - nitro. Examples of +M substituents: alcohol - amine-benzene. The net electron flow from or to the substituent is determined also by the inductive effect. The mesomeric effect as a result of p-orbital overlap (resonance) has absolutely no effect on this inductive effect, as the inductive effect is purely to do with the electronegativity of the atoms and their topology in the molecule (which atoms are connected to which). The concepts of mesomeric effect, mesomerism and mesomer were introduced by Ingold in 1938 as an alternative to the Pauling's synonymous concept of resonance. In chemistry and physics, the 'Inductive Effect' is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule. The net polar effect exerted by a substituent is a combination of this inductive effect and the mesomeric effect. The electron cloud in aσ-bond between two unlike atoms is not uniform and is slightly displaced towards the more electronegative of the two atoms. This causes a permanent state of bond polarization, where the more electronegative atom has a slight negative charge (δ–) and the other atom has a slight positive charge (δ+). If the electronegative atom is then joined to a chain of atoms, usually carbon, the positive charge is relayed to the other atoms in the chain. This is the electron-withdrawing inductive effect, also known as the 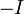 effect. Some groups, such as the alkyl group are less electron-withdrawing than hydrogen and are therefore considered as electron-releasing. This is electron releasing character and is indicated by the
effect. Some groups, such as the alkyl group are less electron-withdrawing than hydrogen and are therefore considered as electron-releasing. This is electron releasing character and is indicated by the  effect. In short alkyl groups are tending to give electrons leading to induction effect. The inductive effect may be caused by some molecules also. Relative inductive effects have been experimentally measured with reference to hydrogen: (Decreasing order of - I effect or increasing order of + I effect).—NR3> —NO2> —SO2R > —CN > —COOH > —F > —Cl> —Br > —I > —OR > —COR > —OH > —C6H5> —CH=CH2> —H. Also the inductive effect is dependent on the distance between the substituent group and the main group that react. That is, as the distance of the substituent group increases the Inductive effect weakens or decreases.
effect. In short alkyl groups are tending to give electrons leading to induction effect. The inductive effect may be caused by some molecules also. Relative inductive effects have been experimentally measured with reference to hydrogen: (Decreasing order of - I effect or increasing order of + I effect).—NR3> —NO2> —SO2R > —CN > —COOH > —F > —Cl> —Br > —I > —OR > —COR > —OH > —C6H5> —CH=CH2> —H. Also the inductive effect is dependent on the distance between the substituent group and the main group that react. That is, as the distance of the substituent group increases the Inductive effect weakens or decreases.
6. Isomerism, types of isomers.Isomerism- the phenomenon ofthe existenceof substances havingthe same molecular formula(ie,having the samequalitative and quantitative composition), but differentphysical and chemical properties. Divided into two: 1.structural.2.stereoisomerism. 1. C5H12.Stereoisomersdiffer inthe spatial arrangement ofatomsin the molecules, i.e. stereoisomershave differentconfiguration.The sequence ofbonds intheir moleculesis the same.
Configuration -the relativespatial arrangement of atomsin a molecule.
The enantiomers-spatialisomerswhose moleculesareto each other asincompatible withthe objectand themirror image ofhim.
(e.g., D-glucose and L-glucose).
Diastereomers-spatialisomers, molecules that do not relateto each otheras a subject, and inconsistent with ita mirror image.
11. Alkyne. Chemical properties.Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic but tend to be more reactive.Chemical properties: Alkynes are characteristically more unsaturated than alkenes. Thus they add two equivalents of bromine whereas an alkene adds only one equivalent. Alkynes are usually more reactive than alkenes. They show greater tendency to polymerize or oligomerize than alkenes do. The resulting polymers, called polyacetylenes are conjugated and can exhibit semiconducting properties. Structure and bonding: In acetylene, the H–C≡C-H bond angles are 180°. By virtue of this bond angle, alkynes tend to be rod-like. Correspondingly, cyclic alkynes are rare. Benzyne is highly unstable. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes or the C-C bond in alkanes .The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol and the second pi-bond of 202 kJ/mol bond strength. Bonding usually discussed in the context of molecular orbital theory, which recognizes the triple bond as arising from overlap of s and p orbitals. In the language of valence bond theory, the carbon atoms in an alkyne bond are sp hybridized: they each have two unhybridizedp orbitals and two sp hybrid orbitals. Overlap of sp orbital from each atom forms one sp-spsigma bond. Each p orbital on one atom overlaps one on the other atom, forming two pi bonds, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent acetylene. The two sp orbitals project on opposite sides of the carbon atom. Hydrogenation to the alkene is usually more desirable since alkanes are less useful:RC≡CR' + H2 → cis-RCH=CR'H. halogenation of alkynes gives the vinyl dihalides or alkyl tetrahalides: RC≡CR' + 2 Br2 → RCBr2CRBr2. RC≡CR + R'C≡CR'  2 RC≡CR'.
2 RC≡CR'.
12. Cycloalkane. Structure, properties.Cycloalkanes are types of alkanes that have one or more rings of carbonatoms in the chemical structure of their molecules. The carbon atoms in cycloalkanes are sp3hybridized and are therefore a deviation from the ideal tetrahedral bond angles of 109°28'. This causes an increase in potential energy and an overall destabilizing effect.Alkanes are types of organichydrocarboncompounds that have only single chemical bonds in their chemical structure. Cycloalkanes consist of only carbon (C) and hydrogen (H) atoms and are saturated because there are no multiple C-C bonds to hydrogenate (add more hydrogen to). A general chemical formula for cycloalkanes would be CnH2(n+1-g) where n = number of C atoms and g = number of rings in the molecule. Cycloalkanes with a single ring are named analogously to their normal alkane counterpart of the same carbon count: cyclopropane, cyclobutane, cyclopentane, cyclohexane, etc. The larger cycloalkanes, with greater than 20 carbon atoms are typically called cycloparaffins. Cycloalkanes are classified into small, common, medium, and large cycloalkanes, where cyclopropane and cyclobutane are the small ones, cyclopentane, cyclohexane, cycloheptane are the common ones, cyclooctane through cyclotridecane are the medium ones, and the rest are the larger ones.Properties: Cycloalkanes are similar to alkanes in their general physical properties, but they have higher boiling points, melting points, and densities than alkanes. Containing only C-C and C-H bonds, unreactivity of cycloalkanes with little or no ring strain are comparable to non-cyclic alkanes.
13. Aromatic compounds. Huckel’s rule.Arenes are hydrocarbons based on the benzene ring as a structural unit. Benzene, toluene, and naphthalene, for example, are arenes. Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Criteria for aromaticity: 1. Cyclic;2. Every atom in ring must be sp^2 hybridized; 3. Every atom in ring needs pure p orbital and ring must be planar; 4. Delocalization of PI electrons, by Huckle'sRule: 4n + 2 = int. The model for benzene consists of two resonance forms, which corresponds to the double and single bonds' switching positions. In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. It was formulated by physical chemist Erich Hückel in 1931. A cyclic ring molecule follows Hückel's rule when the number of its pi electrons equals 4n + 2 where n is zero or any positive integer. Hückel's rule is not valid for many compounds containing more than three fused aromatic nuclei in a cyclic fashion like in pyrene or coronene. Aromatic compounds, originally named because of their fragrant properties, are unsaturatedhydrocarbon ring structures that exhibit special properties due to their aromaticity, including an unusual stability. Aromatic compounds are generally nonpolar and immiscible with water. As they are often unreactive, they are useful as solvents for other nonpolar compounds. Due to their high ratio of carbon to hydrogen, aromatic compounds are characterized by a sooty yellow flame. The double bonds in aromatic compounds are less likely to participate in addition reactions than those found in typical alkenes. Because of pi electrons, aromatic compounds can act as Lewis bases, and they often participate in electrophilic substitution reactions. Aromatic compounds are produced from a variety of sources including petroleum and coal tar. Poly-aromatic hydrocarbons are components of atmospheric pollution and are known carcinogens. Aromatic compounds are also of interest because of their role in the origins of life as precursors to nucleotides and amino acids.Naphthalene is bicyclic (has two rings), and its two benzene rings share a common side. Anthracene and phenanthrene are both tricyclic aromatic hydrocarbons. 
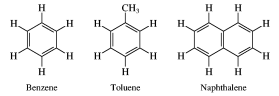
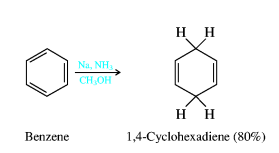
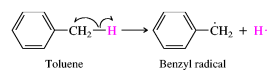

14. Homologous series of benzene. Features of isomerism in benzene. Benzene is the simplest aromatic compound, and it is the first member of the arene homologous series of compounds. Benzene consists of a chain of six carbon atoms in a ring structure, to which are attached six hydrogen atoms. The bonding between the carbon atoms consist of alternating single and double bonds, which have two equivalent arrangements. These equivalent arrangements of bonds are called canonical forms and the overall nature of the bonding is a combination of all canonical form in a resonance hybrid. This bonding between the carbon atoms of benzene gives rise to the aromatic properties of benzene. Aromatic compounds are those possessing the ring structure of benzene (or other molecular structures that resemble benzene in electronic configuration and chemical behaviour). There are many compounds that, at first appearance, bear little resemblance to benzene, but have a basic similarity in electronic configuration. However, at this stage, it is adequate to interpret character in terms of the benzene ring structure, since this definition incorporates most of the commonly encountered aromatic substances. It would be impossible to study each organic compound individually; no one would live long enough. Rather, scientists strive to organise knowledge. The chemist classifies organic compounds by structural and chemical similarities. Simple organic compounds are categorised according to their Functional Groups. These are groups of atoms or bonds common to a series or family of compounds and which decide the principal chemical properties of the series. Such a series is called a Homologous Series, and can be represented by a general formula. Chemical properties homologues of benzene. Homologues of benzene have a number of special chemical properties, connected with mutual influence of alkyl radical on the benzene ring, and Vice versa. Homologous series of benzene meets the General formula Ñ6Í2ï-6. Mono-substituted derivatives of benzene does not have positional isomers, as all the atoms in the benzene nucleus are equal. Di substituted derivatives exist in the form of three isomers,differing mutual location of deputies. The position of Vice-point numbers or prefixes: ortho- (o-), meta- (m-), pair- (p-) 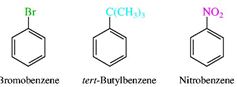
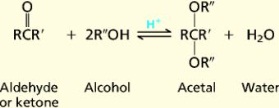
15. Alcohols. Enols.Chemical properties. In chemistry, an alcohol is an organic compound in which the hydroxyl functional group (-OH) is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms. An important class of alcohols are the simple acyclic alcohols, the general formula for which is CnH2n+1OH. Of those, ethanol (C2H5OH) is the type of alcohol found in alcoholic beverages, and in common speech the word alcohol refers specifically to ethanol. Other alcohols are usually described with a clarifying adjective, as in isopropyl alcohol orwood alcohol . For example, the simplest two alcohols are methanol and ethanol ,which have the following structures. In general, the hydroxyl group makes the alcohol molecule polar. Two opposing solubility trends in alcohols are: the tendency of the polar OH to promote solubility in water, and the tendency of the carbon chain to resist it. Thus, methanol, ethanol, and propanol are miscible in water because the hydroxyl group wins out over the short carbon chain. Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons (pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance. All simple alcohols are miscible in organic solvents.Because of hydrogen bonding, alcohols tend to have higher boiling points than comparable hydrocarbons and ethers.Alcohols can also undergo oxidation to give aldehydes, ketones, or carboxylic acids, or they can be dehydrated to alkenes. They can react to form ester compounds, and they can (if activated first) undergo nucleophilic substitution reactions.Enols (also known as alkenols) are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates. The C=C double bond with adjacent alcohol gives enols and enediols their chemical characteristics, by which they present keto-enoltautomerism. In keto-enoltautomerism, enols interconvert with ketones or aldehydes.
 Enol;
Enol;
 Enediol.
Enediol.
16. Phenols. Properties.Mutual influence of the hydroxide and a benzene ring. Phenols are compounds that have a hydroxyl group bonded directly to a benzene or benzenoid ring. C6H5OH. Is important industrial chemical.Difunctionalcompounds;the hydroxyl group and the aromatic ring interact strongly, affecting each other’s reactivity. An old name for benzene was phene, and its hydroxyl derivative came to be called phenol. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (-C6H5) bonded to a hydroxyl group (-OH). It is mildly acidic, but requires careful handling due to its propensity to cause burns. Phenol is appreciably soluble in water, with about 8.3 g dissolving in 100 mL (0.88 M). Phenol is planar with C-O-H angle of 109.Phenol is weakly acidic but at high pH's gives the phenolateanion C6H5O− (also called phenoxide):PhOH  PhO- + H+ K = 10-10 Compared to aliphaticalcohols, phenol is about 1 million times more acidic, although it is still considered a weak acid. It reacts completely with aqueousNaOH to lose H+, whereas most alcohols react only partially. Phenols are less acidic than carboxylic acids, and even carbonic acid. Phenol is highly reactive toward electrophilic aromatic substitution as the oxygen atom's pi electrons donate electron density into the ring. By this general approach, many groups can be appended to the ring, via halogenation, acylation, sulfonation, and other processes. However, phenol's ring is so strongly activated — second only to aniline - that bromination or chlorination of phenol leads to substitution on all carbons ortho and para to the hydroxy group, not only on one carbon. Aqueous solution of phenol is weakly acidic and turns blue litmus slightly to red. Phenol is easily neutralized by sodium hydroxide forming sodium phenate or phenolate, but it being weaker than carbonic acid cannot be neutralized by sodium bicarbonate or sodium carbonate to liberate carbon dioxide C6H5OH + NaOH → C6H5ONa + H2O.When a mixture of phenol and benzoyl chloride when shaken in presence of dilute sodium hydroxide solution, phenyl benzoate is formed. This is an example of Schotten-Baumann reaction:C6H5OH + C6H5COCl → C6H5OCOC6H5 + HCl. Phenol is reduced to benzene when it is distilled with zinc dust or its vapour is passed over granules of zinc at 400 °C:C6H5OH + Zn → C6H6 + ZnO. When phenol is reacted with diazomethane in the presence of boron trifluoride (BF3), anisole is obtained as the main product and nitrogen gas is released:C6H5OH + CH2N2 → C6H5OCH3 + N2. Phenol and its vapors are corrosive to the eyes, the skin, and the respiratory tract. Repeated or prolonged skin contact with phenol may cause dermatitis, or even second and third-degree burns. Inhalation of phenol vapor may cause lung edema. Numbering of the ring begins at hydroxyl-substituted carbon and proceeds in the direction that gives the lower number to the next substituted carbon. Substituents are cited in alphabetical order.
PhO- + H+ K = 10-10 Compared to aliphaticalcohols, phenol is about 1 million times more acidic, although it is still considered a weak acid. It reacts completely with aqueousNaOH to lose H+, whereas most alcohols react only partially. Phenols are less acidic than carboxylic acids, and even carbonic acid. Phenol is highly reactive toward electrophilic aromatic substitution as the oxygen atom's pi electrons donate electron density into the ring. By this general approach, many groups can be appended to the ring, via halogenation, acylation, sulfonation, and other processes. However, phenol's ring is so strongly activated — second only to aniline - that bromination or chlorination of phenol leads to substitution on all carbons ortho and para to the hydroxy group, not only on one carbon. Aqueous solution of phenol is weakly acidic and turns blue litmus slightly to red. Phenol is easily neutralized by sodium hydroxide forming sodium phenate or phenolate, but it being weaker than carbonic acid cannot be neutralized by sodium bicarbonate or sodium carbonate to liberate carbon dioxide C6H5OH + NaOH → C6H5ONa + H2O.When a mixture of phenol and benzoyl chloride when shaken in presence of dilute sodium hydroxide solution, phenyl benzoate is formed. This is an example of Schotten-Baumann reaction:C6H5OH + C6H5COCl → C6H5OCOC6H5 + HCl. Phenol is reduced to benzene when it is distilled with zinc dust or its vapour is passed over granules of zinc at 400 °C:C6H5OH + Zn → C6H6 + ZnO. When phenol is reacted with diazomethane in the presence of boron trifluoride (BF3), anisole is obtained as the main product and nitrogen gas is released:C6H5OH + CH2N2 → C6H5OCH3 + N2. Phenol and its vapors are corrosive to the eyes, the skin, and the respiratory tract. Repeated or prolonged skin contact with phenol may cause dermatitis, or even second and third-degree burns. Inhalation of phenol vapor may cause lung edema. Numbering of the ring begins at hydroxyl-substituted carbon and proceeds in the direction that gives the lower number to the next substituted carbon. Substituents are cited in alphabetical order.
17. The functions of the phenolic compounds.Phenolic acids are plant metabolites widely spread throughout the plant kingdom. Recent interest in phenolic acids stems from their potential protective role, through ingestion of fruits and vegetables, against oxidative damage diseases (coronary heart disease, stroke, and cancers). Phenolic compounds are essential for the growth and reproduction of plants, and are produced as a response for defending injured plants against pathogens. The importance of antioxidant activities of phenolic compounds and their possible usage in processed foods as a natural antioxidant have reached a new high in recent years. Phenolics in Plants. Phenolic acid compounds seem to be universally distributed in plants. They have been the subject of a great number of chemical, biological, agricultural, and medical studies. They form a diverse group that includes the widely distributed hydroxybenzoic and hydroxycinnamic acids. Hydroxycinnamic acid compounds occur most frequently as simple esters with hydroxy carboxylic acids or glucose. Hydroxybenzoic acid compounds are present mainly in the form of glucosides. Content of Phenolic compounds in Tea, Coffee, Berries and Fruits. Besides rowanberry, the best phenolic acid sources among berries are chokeberry, blueberry, sweet rowanberry, and saskatoon berry. Among fruits, the highest contents ared in dark plum, cherry, and one apple variety (ValkeaKuulas). Coffee as well as green and black teas are the best sources among beverages. Caffeic acid dominates in all of these samples except in tea brews.Chemistry.Plant phenolic compounds are diverse in structure but are characterised by hydroxylated aromatic rings (e.g. flavan-3-ols). They are categorised as secondary metabolites, and their function in plants is often poorly understood. Many plant phenolic compounds are polymerised into larger molecules such as the proanthocyanidins (PA; condensed tannins) and lignins. Furthermore, phenolic acids may occur in food plants as esters or glycosides conjugated with other natural compounds such as flavonoids, alcohols, hydroxyfatty acids, sterols, and glucosides. Phenolic compounds. Phenol, the parent compound, used as an disinfectant and for chemical synthesis. Polyphenols like the flavonoids and tannins. Capsaicin, the pungent compound of chilli peppers. Tyrosine, an amino acid.
Salicylic acid is a phenolic compound.Gallic acid, found in gallnuts.Ellagic acid.Natural Substances with Phenolic acids.Propolis is one of the few natural remedies that has maintained its popularity over a long period of time. The pharmacologically active molecules in the propolis are flavonoids and phenolic acids and their esters. These components have multiple effects on bacteria, fungi and viruses. In addition, propolis and its components have anti-inflammatory and immunomodulatory activities. Moreover, propolis has been shown to lower blood pressure and cholesterol levels. Coffee is particularly rich in bound phenolic acids, such as caffeic acid, ferulicacid.Purple cornmaize.Quince has phenolic acids.Aloeferox has phenolic acids.Salicylic acid, a plant hormone and analgesic, antipyretic, and anti-inflammatory drug, precursor compound to Aspirin. Aspirin (acetylsalicylic acid) is still the most commonly used salicylate.
18. Amins, properties and function. Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Compounds with the nitrogen atom attached to a carbonyl of the structure R–CO–NR′R″ are called amides and have different chemical properties from amines. Alkylamines have their nitrogen attached to sp3-hybridized carbon; arylamines have their nitrogen attached to an sp2-hybridized carbon of a benzene or benzene-like ring. 
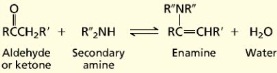
 Amines are classified according to their degree of substitution at nitrogen. An amine with one carbon attached to nitrogen is a primary amine, an amine with two is a secondary amine, and an amine with three is a tertiary amine. Nitrogen's unshared electron pair is of major importance in understanding the structure and properties of amines. Alkylamines have a pyramidal arrangement of bonds to nitrogen, and the unshared electron pair resides in an sp2-hybridized orbital. The geometry at nitrogen in arylamines is somewhat flatter than in alkylamines, and the unshared electron pair is delocalized into the π system of the ring. Delocalization binds the electron pair more strongly in arylamines than in alkylamines. Arylamines are less basic and less nucleophilic than alkylamines. Amines are less polar than alcohols. Hydrogen bonding in amines is weaker than in alcohols because nitrogen is less electronegative than oxygen. Amines have lower boiling points than alcohols, but higher boiling points than alkanes. Primary amines have higher boiling points than isomeric secondary amines; tertiary amines, which cannot form intermolecular hydrogen bonds, have the lowest boiling points. Amines resemble alcohols in their solubility in water. The more basic the amine, the weaker its conjugate acid. The more basic the amine, the larger the pKa of its conjugate acid. The amines obtained from plants to be called alkaloids. The number of known alkaloids exceeds 5000. They are of special interest because most are characterized by a high level of biological activity. Some examples include cocaine, coniine, and morphine.
Amines are classified according to their degree of substitution at nitrogen. An amine with one carbon attached to nitrogen is a primary amine, an amine with two is a secondary amine, and an amine with three is a tertiary amine. Nitrogen's unshared electron pair is of major importance in understanding the structure and properties of amines. Alkylamines have a pyramidal arrangement of bonds to nitrogen, and the unshared electron pair resides in an sp2-hybridized orbital. The geometry at nitrogen in arylamines is somewhat flatter than in alkylamines, and the unshared electron pair is delocalized into the π system of the ring. Delocalization binds the electron pair more strongly in arylamines than in alkylamines. Arylamines are less basic and less nucleophilic than alkylamines. Amines are less polar than alcohols. Hydrogen bonding in amines is weaker than in alcohols because nitrogen is less electronegative than oxygen. Amines have lower boiling points than alcohols, but higher boiling points than alkanes. Primary amines have higher boiling points than isomeric secondary amines; tertiary amines, which cannot form intermolecular hydrogen bonds, have the lowest boiling points. Amines resemble alcohols in their solubility in water. The more basic the amine, the weaker its conjugate acid. The more basic the amine, the larger the pKa of its conjugate acid. The amines obtained from plants to be called alkaloids. The number of known alkaloids exceeds 5000. They are of special interest because most are characterized by a high level of biological activity. Some examples include cocaine, coniine, and morphine. 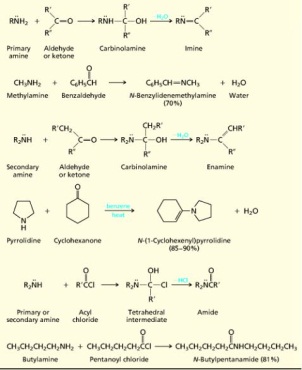
19. Natural carbonyl compounds, function. In organic chemistry, a carbonyl group is a functional group composed of a carbonatomdouble-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups.  . Aldehyde
. Aldehyde 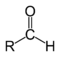 Ketone
Ketone 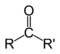 Carboxylic acid
Carboxylic acid 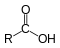 Ester
Ester 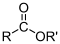 Amide
Amide  .
.  .
.  Two notable aspects of carbonyl group are its geometry and polarity. The coplanar geometry of the bonds to carbonyl group is seen in the molecular models of formaldehyde, acetaldehyde and acetone. The bond angles involving the carbonyl group are 120. Sp2 hybridization.
Two notable aspects of carbonyl group are its geometry and polarity. The coplanar geometry of the bonds to carbonyl group is seen in the molecular models of formaldehyde, acetaldehyde and acetone. The bond angles involving the carbonyl group are 120. Sp2 hybridization. 



 .
.
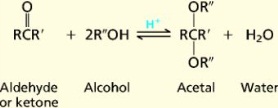
20. Aldehydes. Structure, chemical properties. An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center (a carbon double bonded to oxygen) bonded to hydrogen and an R group, which is any generic alkyl or side chain. The group without R is called the aldehyde group or formyl group. Aldehydes differ from ketones in that the carbonyl is placed at the end of a carbon skeleton rather than between two carbon atoms. Aldehydes are common in organic chemistry. 
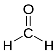 Formaldehyde, the simplest aldehyde. Aldehydes feature an sp2-hybridized, planar carbon center that is connected by a double bond to oxygen and a single bond to hydrogen. The C-H bond is not acidic. Aldehydes (except those without an alpha carbon, or without protons on the alpha carbon, such as formaldehyde and benzaldehyde) can exist in either the keto or the enoltautomer. Keto-enoltautomerism is catalyzed by either acid or base. Usually the enol is the minority tautomer, but it is more reactive. Acyclic aliphatic aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and CH3CH2CH2CHO is named as a derivative of butane. The name is formed by changing the suffix -e of the parent alkane to -al, so that HCHO is named methanal, and CH3CH2CH2CHO is named butanal.If replacing the aldehyde group with a carboxyl group (-COOH) would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix -ic acid or -oic acid in this trivial name by -aldehyde. Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The two aldehydes of greatest importance in industry, formaldehyde and acetaldehyde, have complicated behavior because of their tendency to oligomerize or polymerize. They also tend to hydrate, forming the geminaldiol. A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can de derived. An example is butanedial, which is also called succinaldehyde.
Formaldehyde, the simplest aldehyde. Aldehydes feature an sp2-hybridized, planar carbon center that is connected by a double bond to oxygen and a single bond to hydrogen. The C-H bond is not acidic. Aldehydes (except those without an alpha carbon, or without protons on the alpha carbon, such as formaldehyde and benzaldehyde) can exist in either the keto or the enoltautomer. Keto-enoltautomerism is catalyzed by either acid or base. Usually the enol is the minority tautomer, but it is more reactive. Acyclic aliphatic aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and CH3CH2CH2CHO is named as a derivative of butane. The name is formed by changing the suffix -e of the parent alkane to -al, so that HCHO is named methanal, and CH3CH2CH2CHO is named butanal.If replacing the aldehyde group with a carboxyl group (-COOH) would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix -ic acid or -oic acid in this trivial name by -aldehyde. Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The two aldehydes of greatest importance in industry, formaldehyde and acetaldehyde, have complicated behavior because of their tendency to oligomerize or polymerize. They also tend to hydrate, forming the geminaldiol. A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can de derived. An example is butanedial, which is also called succinaldehyde. 
 ,
,  ,
,  ,
, 
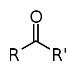 21. Ketones. Structure, chemical properties.
21. Ketones. Structure, chemical properties.
In chemistry, a ketone is an organic compound with the structure RC(=O)R', where R and R' can be a variety of carbon-containing substituents. Ketones feature a carbonyl group (C=O) bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology. Examples include many sugars (ketoses) and the industrial solvent acetone. According to the rules of IUPAC nomenclature, ketones are named by changing the suffix -ane of the parent alkane to -anone. For the most important ketones, however, traditional nonsystematic names are still generally used, for example acetone and benzophenone. The ketone carbon is often described as "sp2 hybridized," a description that includes both their electronic and molecular structure. Ketones are trigonal planar around the ketonic carbon, with C-C-O and C-C-C bond angles of approximately 120°. Ketones differ from aldehydes in that the carbonyl group (CO) is bonded to two carbons within a carbon skeleton. In aldehydes, the carbonyl is bonded to one carbon and one hydrogen and are located at the ends of carbon chains. Ketones are also distinct from other carbonyl-containing functional groups, such as carboxylic acids, esters and amides. Many methods exist for the preparation ofketones. In industry, the most important method probably involves oxidation of hydrocarbons, often with air. For example, a billion kilograms of cyclohexanone are produced annually by aerobic oxidation of cyclohexane. Acetone is prepared by air-oxidation of cumene. For specialized or small scale organic synthetic applications, ketones are often prepared by oxidation of secondary alcohols:R2CH(OH) + O → R2C=O + H2O.
 22. Carboxylic acids. Classification, chemical properties.
22. Carboxylic acids. Classification, chemical properties.
A carboxylic acid is an organic acid characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH. A carboxyl group (or carboxy) is a functional group consisting of a carbonyl (RR'C=O) and a hydroxyl(R-O-H), which has the formula -C(=O)OH, usually written as -COOH or -CO2H.
Carboxylic acids are Brønsted-Lowry acids because they are proton (H+) donors. They are the most common type of organic acid. Among the simplest examples are formic acid H-COOH, which occurs in ants, and acetic acid CH3-COOH, which gives vinegar its sour taste. Acids with two or more carboxyl groups are called dicarboxylic, tricarboxylic, etc. The simplest dicarboxylic example is oxalic acid (COOH)2, which is just two connected carboxyls.Salts and esters of carboxylic acids are called carboxylates. When a carboxyl group is deprotonated, its conjugate base forms a carboxylate anion. Carboxylate ions are resonance stabilized and this increased stability makes carboxylic acids more acidic than alcohols. Carboxylic under some circumstances can be decarboxylated to yield carbon dioxide.Carboxylic acids are polar. Because they are both hydrogen-bond acceptors (the carbonyl) and hydrogen-bond donors (the hydroxyl), they also participate in hydrogen bonding. The carboxylate anion R-COO– is usually named with the suffix -ate, so acetic acid, for example, becomes acetate ion. In IUPAC nomenclature, carboxylic acids have an -oic acid suffix (e.g., octadecanoic acid). In common nomenclature, the suffix is usually -ic acid (e.g., stearic acid).
23. Derivatives of carboxylic acids.
The carboxyl group (abbreviated -CO2H or -COOH) is one of the most widely occurring functional groups in chemistry as well as biochemistry. The carboxyl group of a large family of related compounds called Acyl compounds or Carboxylic Acid Derivatives.All the reactions and compounds covered in this section will yield Carboxylic Acids on hydrolysis, and thus are known as Carboxylic Acid Derivatives. Hydrolysis is one example of Nucleophilic Acyl Substitution, which is a very important two step mechanism that is common in all reactions that will be covered here.Structure.This group of compounds also contains a carbonyl group, but now there is an electronegative atom (oxygen, nitrogen, or a halogen) attached to the carbonyl carbon. This difference in structure leads to a major change in reactivity.The systematic IUPAC nomenclature for carboxylic acid derivatives is different for the various compounds which are in this vast category, but each is based upon the name of the carboxylic acid closest to the derivative in structure. Acyl Groups.Acyl groups are named by stripping the -ic acid of the corresponding carboxylic acid and replacing it with -yl.EXAMPLE:CH3COOH = aceticacid.CH3COO-R = acetyl-R. Acyl Halides.Simply add the name of the attached halide to the end of the acyl group.EXAMPLE:CH3COOH = aceticacid.CH3COBr = acetyl bromide. CarboxylicAcidAnhydride.A carboxylic acid anhydride ([RC=O]O[O=CR]) is a carboxylic acid (COOH) that has an acyl group (RC=O) attached to its oxygen instead of a hydrogen. If both acyl groups are the same, then it is simply the name of the carboxylic acid withtheword acid replaced with anhydride. If the acyl groups are different, then they are named in alphabetical order in the sameway,with anhydridereplacing acid.EXAMPLE:
CH3COOH = acetic acid
CH3CO-O-OCCH3 = EthanoicAnhydride.Esters.Esters are created when the hydrogen on a carboxylic acid is replaced by an alkyl group. Esters are known for their pleseant, fruity smell and taste, and they are often found in both natural and artificial flavors. Esters (RCOOR1) are named as alkyl alkanoates. The alkyl group directly attached to the oxygen is named first, followed by the acyl group, with -ate replacing -yl of the acyl group.EXAMPLE:
CH3COOH = acetic acid
CH3COOCH2CH2CH2CH3 = acetyl butanoate. Amides.Amides which have an amino group (-NH2) attached to a carbonyl group (RC=O) are named by replacing the -oic acid or -ic acid of the corresponding carboxylic acid with -amide.EXAMPLE:
CH3COOH = acetic acid
CH3CONH2 = acetamide.Nitriles.Nitriles (RCN) can be viewed a nitrogen analogue of a carbonyl and are known for their strong electron withdrawing nature and toxicity. Nitriles are named by adding the suffix -nitrile to the longest hydrocarbon chain (including the carbon of the cyano group). It can also be named by replacing the -ic acid or -oic acid of their corresponding carboxylic acids with -onitrile. Functional class IUPAC nomenclature may also be used in the form of alkyl cyanides.EXAMPLE:CH3CH2CH2CH2CN= butonitrile or butyl cyanide.
24. Unsaturated and aromatic acids. Structure, chemical properties.Unsaturated acids are acids of ethylene series of general formula C n H 2n _ 2O2and acids of the general formula C n H 2n – 4 O2. Such composition may contain either one acetylenicortwoethylenicbond.Monobasic acid of ethylene series usually has trivial names. First homolog CH2 = CH-COOH, said acrylic acid of propenoic acid. Second homologue may exist in three structurally isomeric forms: 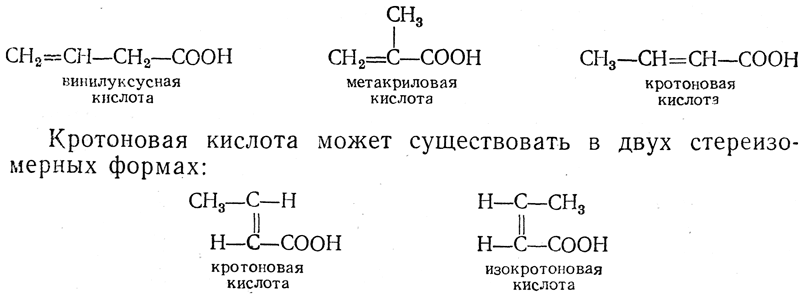


They form acid derivatives used to make polymers such as polymethyl methacrylate used for the production of organic glass.
Higher unsaturated acids are part of the liquid vegetable fats. This oleic acid (one double bond) C17H33COOH, linoleic acid (two double bonds) C17H31COOH, and linolenic (three double bonds) C17H29COOH acid. Biologically they are active only in the cys-form.

Cys-oleic acid.Unsaturateddicarboxylic acids may also have geometrical isomerism, while isomers have not only different but also some physical chemical properties. It acids such as maleic acid (cis-butenoic acid), fumaric acid (trans-butenoic acid). 


as maleic acid (cis-butenoic acid) 

 fumaric acid (trans-butenoic acid). Aromatic acids.Aromatic carboxylic acids are called benzene derivatives containing carboxyl groups directly linked to carbon atoms of the benzene nucleus. Acids containing carboxyl groups in the side chains are regarded as aliphatic-aromatic.The most important representatives of the aromatic acids are benzoic and phthalic acid.Benzoic acid C6H5COOH has antiseptic properties, is contained in cranberries, lingonberries.Benzoic acid hasn’t isomers. The second member of the homologous series may have already 4 isomers:
fumaric acid (trans-butenoic acid). Aromatic acids.Aromatic carboxylic acids are called benzene derivatives containing carboxyl groups directly linked to carbon atoms of the benzene nucleus. Acids containing carboxyl groups in the side chains are regarded as aliphatic-aromatic.The most important representatives of the aromatic acids are benzoic and phthalic acid.Benzoic acid C6H5COOH has antiseptic properties, is contained in cranberries, lingonberries.Benzoic acid hasn’t isomers. The second member of the homologous series may have already 4 isomers: 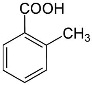 orto-methylbenzoic acid.
orto-methylbenzoic acid.  Meta-methylbenzoic acid.
Meta-methylbenzoic acid. 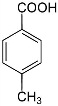 Para-methylbenzoic acid.Aromatic acids enter all those reactions that are typical and fatty acids: dissociation and the formation of salts, anhydrides, amides, acid halides, esters, decarboxylation. But aromatic acids greater degree of dissociation as a benzene ring possesses electrophilic character and, pulling the electron density on itself, reduces hydrogen bond with a carboxyl group.
Para-methylbenzoic acid.Aromatic acids enter all those reactions that are typical and fatty acids: dissociation and the formation of salts, anhydrides, amides, acid halides, esters, decarboxylation. But aromatic acids greater degree of dissociation as a benzene ring possesses electrophilic character and, pulling the electron density on itself, reduces hydrogen bond with a carboxyl group.
25. Heterofunctional compounds.Heterofunctional compounds contain per molecule two or more different functional groups. These compounds are biologically important compounds, many participants in the processes occurring in living organisms, as well as E-medicinal drugs.
To heterofunctional compounds include aminoalcohols, hydroxy, amino, oxo acids, vitamins, hormones, coenzymes, etc.
The chemical properties of heterofunctional compounds largely determined by properties of the respective mono-functional derivatives. Therefore, there is some similarity in behavior, ie ability to react each functional group. However, the simultaneous presence of several functional groups in a molecule leads to some differences, such as contrast enhancement or attenuation of some properties. For example, the accumulation of acid groups increases the acidic properties of the compounds, and the accumulation of basic amionogrupp in the molecule increases the basicity. Heterofunctional compound containing both acidic and basic functional groups, such as amino acids, exhibit amphoteric properties, i.e. capable of reacting with acids as well as with bases.Aminoalcohols contain OH and NH2 groups, for example, .2-aminoethanol (colamine) included in the phospholipids.
Hydroxy (hydroxy) contain OH and COOH groups, for example, . hydroxypropanoic (lactic acid). Produced during anaerobic glycolysis.
Oxoacids (keto) containing C = O and COOH groups. For instance, . 2 oxopropanoic (pyruvic acid). Pyruvic acid formed by oxidation of lactic acid is an intermediate in the metabolism of carbohydrates.
Amino acids contain NH2 and COOH groups. For instance, . 2-aminopropanoic acid (alanine). Alanine is a member of peptides and proteins.In all of the aliphatic series have a functional group and an electron acceptor character, shifting the electron density on themselves, contribute to the reactivity of each of the functional groups.For example, the oxo acid, electrophilicity of each of the two carbonyl carbon atoms increases under the influence of a negative inductive effect, another functional group which increases the reactivity of:
As the inductive effect fades in 3-4 ties, the important factor is the relative position of the functional groups in the carbon chain. Functional groups may be one and the same carbon atom (α-location) or at different (β -, g-, d - location, etc.)


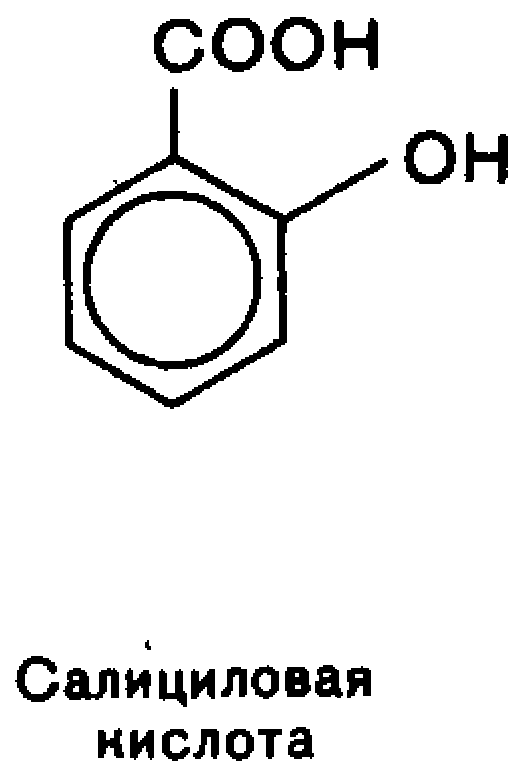
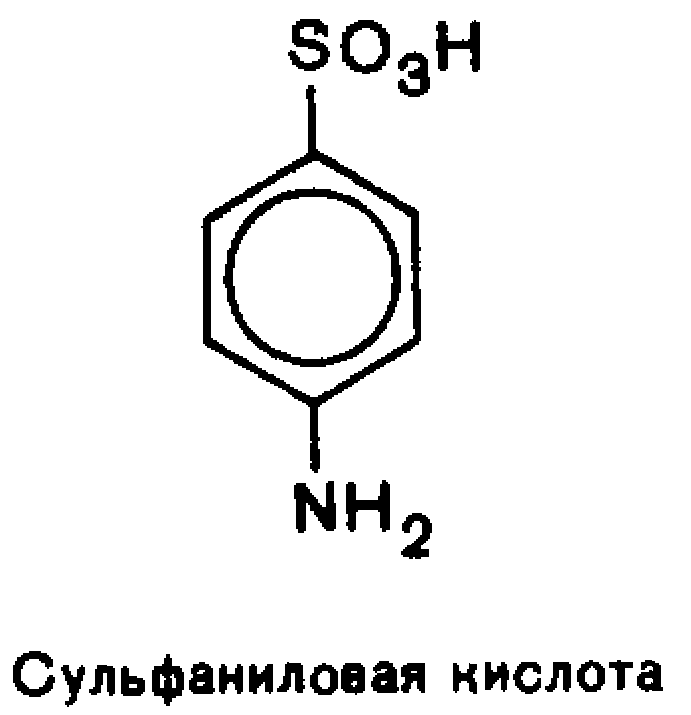
 Each of the functional group retains its own reactivity, which is enhanced under the influence of the other.In aromatic series based important biologically active compounds and synthetic drugs are n-aminophenol, n-aminobenzoic, salicylic and sulfanilicacids.
Each of the functional group retains its own reactivity, which is enhanced under the influence of the other.In aromatic series based important biologically active compounds and synthetic drugs are n-aminophenol, n-aminobenzoic, salicylic and sulfanilicacids. 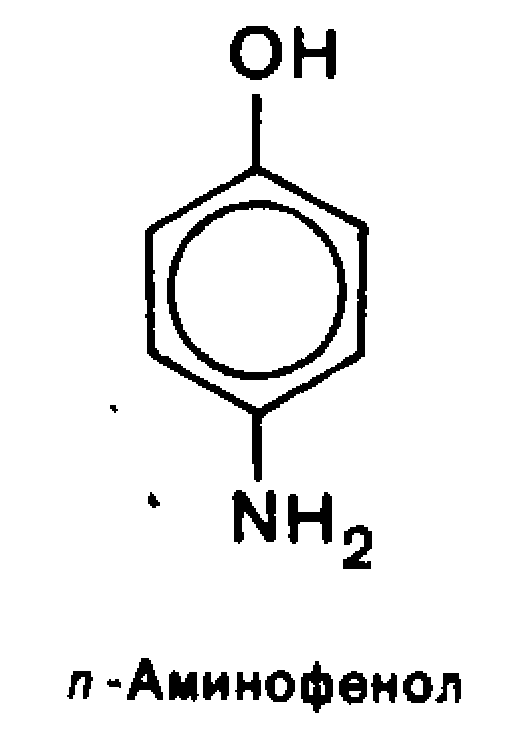

26. Hydroxy acid. Structure, chemical properties.α-Hydroxy acids, or alpha hydroxy acids (AHAs), are a class of chemical compounds that consist of a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. They may be either naturally occurring or synthetic. AHAs are well known for their use in the cosmetics industry. They are often found in products claiming to reduce wrinkles or the signs of aging, and improve the overall look and feel of the skin. They are also used as chemical peels available in a dermatologist's office, beauty and health spas and home kits, which usually contain a lower concentration of around 4%. Although their effectiveness is documentednumerous cosmetic products have appeared on the market with unfounded claims of performance. Many well-known α-hydroxy acids are useful building blocks in organic synthesis: the most common and simple are glycolic acid, lactic acid, citric acid, mandelic acid.Chemical acidity.Although these compounds are related to the ordinary carboxylic acids, and therefore are weak acids, their chemical structure allows for the formation of an internal hydrogen bond between the hydrogen at the hydroxyl group and one of the oxygen atoms of the carboxylic group. Two effects emerge from this situation:Due to the "occupation" of electrons of the carboxylic oxygens in the hydrogen bonding, the acidic proton is held less strongly, as the same electrons are used in bonding that hydrogen too. So the pKa of 2-hydroxypropanoic acid (lactic acid) is a full unit lower compared to that of propionic acid itself. The internal bridging hydrogen is locked in its place on the NMR timescale: in mandelic acid (2-hydroxy-2-phenylacetic acid) this proton couples to the one on carbon in the same way and magnitude as hydrogens on geminal carbon atoms.A beta hydroxy acid or β-hydroxy acid (BHA) is an organic compound that contains a carboxylic acid functional group and hydroxy functional group separated by two carbon atoms. They are closely related to alpha hydroxy acids, in which the two functional groups are separated by one carbon atom.In cosmetics, the term beta hydroxy acid refers specifically to salicylic acid, which is used in some "anti-aging" creams and acne treatments.Upon dehydration, beta-hydroxy acids yield an alpha-beta unsaturated acid. Acidic properties.Compared to non-hydroxylated carboxylic acids, this group of acids is stronger, although less strong than the alpha hydroxy acids. Due to the larger distance, the intramolecular hydrogen bridge is less easily formed compared to the alpha hydroxy acids. The table summarizes some values on the propionic series. 
27. Aromatic hydroxy acids. Salicylic acid.Benzoic acid C7H6O2 (or C6H5COOH), is a colorless crystalline solid and a simple aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates. 
Benzoic acid is produced commercially by partial oxidation of toluene with oxygen. The process is catalyzed by cobalt or manganese naphthenates. The process uses cheap raw materials, proceeds in high yield, and is considered environmentally green. 
Benzoic acid is mainly consumed in the production of phenol by oxidative decarboxylation at 300−400 °C:C6H5CO2H + 1/2 O2 → C6H5OH + CO2. The temperature required can be lowered to 200 °C by the addition of catalytic amounts of copper(II) salts. The phenol can be converted to cyclohexanol, which is a starting material for nylon synthesis.Benzoate plasticizers, such as the glycol-, diethylenegylcol-, and triethyleneglycol esters, are obtained by transesterification of methyl benzoate with the corresponding diol. Alternatively these species arise by treatment of benzoylchloride with the diol. These plasticizers are used similarly to those derived from terephthalic acid ester.Benzoic acid and its salts are used as a food preservatives, represented by the E-numbers. Benzoic acid inhibits the growth of mold, yeast and some bacteria. It is either added directly or created from reactions with its sodium, potassium, or calcium salt. The mechanism starts with the absorption of benzoic acid in to the cell. Acidic food and beverage like fruit juice (citric acid), sparkling drinks (carbon dioxide), soft drinks (phosphoric acid), pickles (vinegar) or other acidified food are preserved with benzoic acid and benzoates.Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century.Salicylic acid is a monohydroxybenzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.
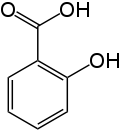 Salicylic acid has the formula C6H4(OH)COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzoic acid. It is poorly soluble in water. Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.Salicylic acid is known for its ability to ease aches and pains and reduce fevers. These medicinal properties, particularly fever relief, have been known since ancient times, and it is used as an anti-inflammatory drug.In modern medicine, salicylic acid and its derivatives are used as constituents of some rubefacient products. For example, methyl salicylate is used as a liniment to soothe joint and muscle pain, and choline salicylate is used topically to relieve the pain of mouth ulcers.
Salicylic acid has the formula C6H4(OH)COOH, where the OH group is ortho to the carboxyl group. It is also known as 2-hydroxybenzoic acid. It is poorly soluble in water. Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.Salicylic acid is known for its ability to ease aches and pains and reduce fevers. These medicinal properties, particularly fever relief, have been known since ancient times, and it is used as an anti-inflammatory drug.In modern medicine, salicylic acid and its derivatives are used as constituents of some rubefacient products. For example, methyl salicylate is used as a liniment to soothe joint and muscle pain, and choline salicylate is used topically to relieve the pain of mouth ulcers.
28. Amino alcohol. Structure, chemical properties.Alkanolamines are chemical compounds that carry hydroxy (-OH) and amino (-NH2, -NHR, and -NR2) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.Amino alcohols are organic compounds that contain both an amine functional group and an alcohol functional group.Ethanolamines 
29. Amino acids. Classification, functions.Classification of Amino Acids.Although there are many ways to classify amino acids, these molecules can be assorted into six main groups, on the basis of their structure and the general chemical characteristics of their R groups. 
In humans, non-protein amino acids also have important roles as metabolic intermediates, such as in the biosynthesis of the neurotransmitter gamma-aminobutyric acid. Many amino acids are used to synthesize other molecules. Some non-standard amino acids are used as defenses against herbivores in plants. The 20 amino acids encoded directly by the genetic code can be divided into several groups based on their properties. Important factors are charge, hydrophilicity or hydrophobicity, size, and functional groups. These properties are important for protein structure and protein–protein interactions. Some amino acids have special properties such as cysteine, that can form covalent disulfide bonds to other cysteine residues, proline that forms a cycle to the polypeptide backbone, and glycine that is more flexible than other amino acids.
30. Aldehyde-and Keto acid. General and special properties.Acetaldehyde (systematically ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part of their normal metabolism. It is also produced by oxidation of ethylene and is popularly believed to be a cause of hangovers from alcohol. 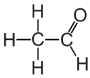 In the liver, the enzyme alcohol dehydrogenase oxidizes ethanol into acetaldehyde, which is then further oxidized into harmless acetic acid by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of NAD+ to NADH.In the brain, alcohol dehydrogenase has a minor role in the oxidation of ethanol to acetaldehyde. Instead, the enzyme catalase primarily oxidizes ethanol to acetaldehyde. The last steps of alcoholic fermentation in bacteria, plants and yeast involve the conversion of pyruvate into acetaldehyde and carbon dioxide by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehy
In the liver, the enzyme alcohol dehydrogenase oxidizes ethanol into acetaldehyde, which is then further oxidized into harmless acetic acid by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of NAD+ to NADH.In the brain, alcohol dehydrogenase has a minor role in the oxidation of ethanol to acetaldehyde. Instead, the enzyme catalase primarily oxidizes ethanol to acetaldehyde. The last steps of alcoholic fermentation in bacteria, plants and yeast involve the conversion of pyruvate into acetaldehyde and carbon dioxide by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehy
Date: 2015-01-29; view: 9320
| <== previous page | | | next page ==> |
| EBM DESIGN Ltd. | | | Topic: Cinema and Theatre in our life. |