
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Kinetic Resolution of Racemic Allylic Alcohols by Enantioselective Epoxidation. A rout to Substances of Absolute Enantiomeric Purity?
The pinacol rearrangement has long been known to be difficult to control in terms of regioselectivity and stereoselectivity. Retention of the configuration is observed in the pinacol-type rearrangement of 2,3-epoxy alcohols 1 in the presence of bis(iodozincio)methane (2). The 1,3-migration of the hydroxymethyl group affords an intermediate 2-hydroxyaldehyde, which is methylenated by 2 in situ to give homoallyl alcohol 3. What about the inversion with methylaluminum bis(4-bromo-
2,6-di-tert-butylphenoxide? Investigated the universality of the reaction and determined the impact of substituents on the quality of its passage. Also one possible mechanism of the reaction was proposed.
Bold numbers, remove temperature, specify R
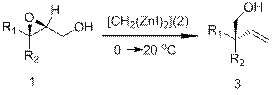
Kinetic Resolution of Racemic Allylic Alcohols by Enantioselective Epoxidation. A rout to Substances of Absolute Enantiomeric Purity?
Abstract:
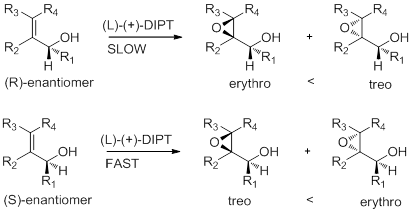
For the introduction of chirality into prochiral allylic alcohols we used a titanium alkoxide tartrate epoxidation (DIPT???) catalyst which is highly effective. It was found that increasing the size of the alkyl group in the tartrate ester significantly increases the rate difference for epoxidation of the S and R enantiomers. The enantiomeric excess realized in an asymmetric synthesis is simply a consequence of the energy difference between two diastereomeric transition states the only way to improve the % ee is to increase that energy difference The energy difference represents a constant and unrelenting differential pressure upon the two enantiomers. (trivial);. So we continued this winnowing until the last molecule of the more reactive enantiomer is swept away, and one is left with a substance possessed of absolute enantiometric purity. This is quite usual approach. Instead, say which R allows to obtain highest ee.
Graphical abstract: give ee intervals, simplify
Date: 2014-12-22; view: 1630