
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Nitrogen
Its electronic formula is 1s22s22p3. On the basis of graphic drawing of the external energy level (2s22p3) the following valency state and oxidation numbers are possible for Nitrogen:
| 2p | ||||||||||
| 2s | ↑ | ↑ | ↑ | |||||||
| ↑↓ | ||||||||||
| 1s | ||||||||||
| ↑↓ | ||||||||||
Valency is III (owing to three unpaired electrons of 2p-sublevel). Valency is IV (three - owing to unpaired 2p-electrons, the forth bond - after donor-acceptor mechanism using 2s-electrons). Oxidation numbers are 3-, 2-, 1-, 0, 1+, 2+, 3+, 4+, 5+.
Nitrogen is the typical non-metal, only Fluorine and Oxygen have stronger electronegativity (3,0). The most widespread Nitrogen compounds of different oxidation levels (from 3- to 5+) are shown in Table 17.
Ammonia (NH3) is colorless gas with purgen odor. In the laboratory heating of NH4Cl with Calcium hydroxide or any strong alkalis can produce it:
2 NH4Cl + Ca(OH)2 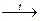 2 NH3↑+ CaCl2 + 2 H2O.
2 NH3↑+ CaCl2 + 2 H2O.
Ammonia can react with water and acids, showing the main properties in the reactions. Practically it does not make NH4OH hydroxide well dissolved in water (31% at 200C), but reacts with H2O by means of Hydrogen bond:
Table 17. General characteristic of Nitrogen compounds
| Oxidation numbers | 3- | 2- | 1- | 1+ | 2+ | 3+ | 4+ | 5+ | |
| Nitrogen compounds | NH3 (NH4+) | N2H4 - hydrazine | NH2OH - hydroxyl-amine | N2 | N2O -laughing gas | NO (N2O2) | N2O3 | NO2 (N2O4) | N2O5 |
| Properties in reactions without oxidation number | Weak basic properties; Proton acceptor | Basic properties; Proton acceptor | Weak base | Low chemical activity | Indifferent oxides | Acid oxides | |||
| HNO2 | HNO2 + HNO3 | HNO3 | |||||||
| Properties in reactions with oxidation number change | Reducing agent only | Reduction properties prevail over oxidation ones | Oxidizing agent; reducing agent relative to F2 and O2 | Oxidizing and reducing agents (depending on reaction conditions) | Oxidizing agent only |
NH3 + H2O ↔ NH3·H2O;
NH3·H2O ↔ NH4OH.
The following important fertilizers are produced by ammonia interaction with acids:
NH3 + HNO3 = NH4NO3 - Ammonia Nitrate;
2NH3 + H2SO4 = (NH4)2SO4 - Ammonia Sulfate;
NH3 + H3PO4 = NH4H2PO4 - Ammonia dihydrophosphate (ammophos);
2NH3 + H3PO4 = (NH4)2HPO4 - Ammonia hydrophosphate (diammophos).
Nitric oxide (NO) is get by oxidation of Ammonia and it is used as the intermediate product in HNO3 production:
4 NH3 + 5 O2 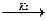 4 NO + 6 H2O
4 NO + 6 H2O
O20 + 4  ® 2 O2- ® 2 O2-
| 5 (reduction); |
N3- - 5  ® N2+ ® N2+
| 4 (oxidation). |
Nitrogen oxide (III) and nitrous acid
Nitrogen oxide (III) is nitrous acid anhydride:
N2O3 + H2O ↔ 2HNO2.
As acid oxide it reacts with alkalis:
N2O3 + 2NaOH = 2NaNO2 + H2O.
Nitrous acid (HNO2) dissociates with Hydrogen ions formation:
HNO2 ↔ H+ + NO2- .
At it’s heating and under the influence of strong acids the process of disproportion takes place:
3HNO2 ↔ HNO3 + 2NO + H2O
N3+ +  ® N2+ ® N2+
| 2 (reduction); |
N3+ - 2  ® N5+ ® N5+
| 1 (oxidation). |
Nitrous acid and its salts can be both reducing and oxidizing agents:
2NaNO2 + 2KI + 2H2SO4 = I2 + 2NO + K2SO4 + Na2SO4 + 2H2O
N3+ + 1  ® N2+ ® N2+
| 2 (reduction); |
2I- - 2  ® I20 ® I20
| 1 (oxidation). |
5KNO2 + 2KMnO4 + 3H2SO4 = 5KNO3 + 2MnSO4+ K2SO4 + 3H2O
Mn7+ + 5  ® Mn2+ ® Mn2+
| 2 (reduction); |
N3+- - 2  ® N5+ ® N5+
| 5 (oxidation). |
Nitric oxide (V) and nitric acid
Nitric acid and its salts (Nitrates) have the oxidizing properties:
3KNO3 + 8Al + 5KOH + 2H2O = 3NH3 + 8KAlO2
N5+ + 8  ® N3- ® N3-
| 3 (reduction); |
Al0 - 3  ® Al3+ ® Al3+
| 8 (oxidation). |
Particular feature of nitric acid is in interaction almost with all metals and non-metals, at the same time it oxidizes them. The reduction of N(5+) but not H(1+) as with acids-oxidizing agents always takes place. Generally NO and NO2 prevail among reduction products. Active metals (Mg, Zn, Ca and others) reduce diluted HNO3 up to N2 and NH4NO3.
At the same time some N(5+) reduction products can be isolated. However, the equations of such reactions are relative and only one compound (NO2, NO or N2, NH3), formed in quantity, is indicated in the products:
4 Mg + 10HNO3 (diluted) = 4Mg(NO3)2 + NH4NO3 + 3H2O;
Cu + 4HNO3 (conc) = Cu(NO3)2 + 2NO2 + 2H2O;
S + 6HNO3 (conc) = H2SO4 + 6NO2 + 2H2O;
3P + 5HNO3 (diluted) + 2H2O = 3H3PO4 + 5NO.
Nitric acid salts - Sodium, Potassium, ammonia, and calcium Nitrates are of great practical value. They are called saltpeters and are used in great quantity as fertililizers.
Date: 2015-01-12; view: 979
| <== previous page | | | next page ==> |
| Experiment 9. Instability of thiosulfuric acid | | | Phosphorus |