
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Experiment 1. Reducing properties of metal ions of lower oxidation number
Add 1-2 drops of Iron (III) Sulfate Fe2(SO4)3 to 2-3 drops of Ammonium Thiocyanate NH4SCN. Register color of solution. Further add to the same tube the solution of Tin (II) Chloride SnCl2 (by drops) up to whole decoloration of mixture. Draw up equations of reactions. For Redox reaction write the scheme of electron balance, and mark oxidizing and reducing agents.
Experiment 2. Influence of medium to redox reactions
Put 2-3 drops of Potassium Permanganate solution KMnO4 in the 3 tubes. Add 1-2 drops of diluted Sulfuric acid to the first tube; - 5-10 drops of distillated water to the second one; 2-3 drops of alkali solution (NaOH or KOH) in the third one. Is the color of KMnO4 solution changed? Then add in each tube few crystals of Sodium Sulfite or Potassium Sulfite. What do you observe? Draw up equations of reactions, write the scheme of electron balance, mark oxidizing and reducing agents.
Experiment 3. Redox properties of Chromium compounds
A) Put 2-3 drops of Potassium Dichromate K2Cr2O7 solution in to the tube, add 2-3 drops of diluted Sulfuric acid and few crystals of solid Sodium Sulfite or Potassium Sulfite. What do you observe? Draw up equations of reactions, write the scheme of electron balance, mark oxidizing and reducing agents.
B) Put 1-2 drops of alkali solution (NaOH or KOH), add by drops the solution of Chromium (III) Sulfate Cr2(SO4)3 up to the formation of precipitate. Register the color of mixture. Further add alkali solution (NaOH or KOH) to the same tube up to the dissolving of precipitate (register the color of solution), then - 3-4 drops of 10% solution of Hydrogen Peroxide H2O2. Heat a content of tube during 2-3 minutes in water bath. How is the a color of solution changed? Draw up equations of reactions of corresponded transformations: Cr3+ → Cr(OH)3↓ → CrO2- → CrO42-. For Redox reaction write the scheme of electron balance, mark oxidizing and reducing agents.
CHAPTER # 10. COMPLEX (COORDINATION) COMPOUNDS
1. Introduction in General, Organic and Biochemistry, 7th Edition, by Morris Hein, Leo R. Best, Scott Pattison and Susan Arena, Brooks/Cole Publishing Co., 2001. (Chapter 25, pp. 733-736);
2. http://library.thinquest.org/3659/trametal.html
3. http://www.hrw.com/science/mc/index/htm
4. http://chemcourse.8m.com/bioeffects.html
1. General characteristics
All the variety of inorganic compounds can be divided into two groups: 1) second order compounds or simple; 2) the highest order compounds.
The substances whose value of element oxidation number coincides with presented valency or with quantity of chemical bonds in molecule (NH3, H2O, SO3, AgCl, etc.) are called simple compounds but the substances in which additional valence bonds occur are considered as compounds of the highest order and are called coordinate or complex.
The simple compounds are structured at the account of ionic, covalent polar or covalent non-polar bond. In contrast to this the additional covalent bond is formed in complex compounds on the basis of donor-acceptor interaction. Owing to this bond the complex bond is formed. Donor-acceptor interaction between central ion-complexing agent and ligands takes place. At the same time ion-complexing agent takes part in formation of the bonds, giving free orbitals of their external and pre-external energy levels but ligands - giving electron pairs of one of the atoms. For example, the following stages can be presented in case of [Co(NH3)6]3+ complex ion formation:
1) Co3+ ion formation from cobalt atom:
- 3 
27Co0 1s22s22p63s23p63d74s24p0 → Co3+ 1s22s22p63s23p63d64s04p0
| 3d | 4s | 4p | Co0 | |||||||||
| ↑↓ | ↑↓ | ↑ | ↑ | ↑ | ↑↓ | → | ||||||
| → Co3+ | 3d | 4s | 4p | ||||||||
| ↑↓ | ↑↓ | ↑↓ | |||||||||
2) ammonia molecule formation:
+ 3  - 1
- 1 
7N0 1s22s22p3 → N3- 1s22s22p6; 1H0 1s1 → H1+ 1s0 or
| 1s | 1s | 1s | ||||||||||||||
| 3H | ↑ | ↑ | ↑ | 
| ||||||||||||
| → | NH3 | ↑↓ | ↑↓ | ↑↓ | ↑↓ | |||||||||||
| 2s | 2p | |||||||||||||||
| N | ↑↓ | ↑ | ↑ | ↑ |
3) formation of [Co(NH3)6]+3 complex ion at the account of six free orbitals 3d, 4s and 4p of Co+3 sublevels and paired Nitrogen 2s electrons which is the part of ammonia six molecules:
| Co+3 | 3d | 4s |  4p 4p
| ||||||||
| ↑↓ | ↑↓ | ↑↓ | |||||||||

| |||||||||||
| 2s | 2p + 1s | ||||||||||
| 6NH3 | ↑↓ | ↑↓ | ↑↓ | ↑↓ |
| [Co(NH3)6]3+ | |||||||||||
 3d 3d
| |||||||||||
| ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑↓ | ↑↓ | |||
2s + 3d+4s+4p
The main principles of complex compound formation were presented in 1893 by Swiss chemist Alfred Werner as coordination theory:
1. In the molecule of any complex compound one of the ions takes the central place and is called complexing agent or central ion;
2. Some quantity of ions or neutral molecules with opposite charges is placed close enough around the central ion and is called ligands.
Central ion with ligands placed around it forms so-called inner coordination sphere of compound (complex ion);
3. Ions, which are located far from central ion, make outer coordination sphere of complex compound;
4. Number, which shows how many ligands are located around complexing agent in the inner sphere, is called coordinating.
Structure of complex compound may be presented as:
| Complexing agent | |||

| |||
  [Cu(NH3)4]SO4 [Cu(NH3)4]SO4
|  Coordination number Coordination number
| ||

| |||
| Ligands, addends |
| Outer sphere |
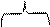
|
| [Cu(NH3)4]SO4 |
|
| Inner sphere |
Date: 2015-01-12; view: 1507
| <== previous page | | | next page ==> |
| Electromotive Series of Metals | | | Rules for naming of coordination compounds |