
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
CHEMISTRY
1 Complete the text about chemistry using the words in the box.
| bind | charge | science |
| nucleus | interactions | shape |
| mass | particles | matter |
| density | substances | amounts |
| ions | molecules |
Chemistry is the______________ that systematically studies the composition, properties and activity of _______________ and various elementary forms of________________ .
Chemistry is the study of matter and energy and interactions between them.
Energy has no _____________ or form. Matter is everything that occupies space and has
____________ .______________ refers to the amount of matter in a given amount of space and is defined as the mass per unit of a substance.
The fundamental building block of matter is the atom. It has a_______________ at its centre consisting of protons, which have a positive electrical __________, and neutrons which are uncharged. Negatively charged electrons circle around nuclei. There are super-small ______________ inside the protons and neutrons called quarks.
Chemical reactions involve______________________ between the electrons of one atom and the electrons of another atom. Atoms which have different _____________ of electrons and protons have positive or negative electrical charge and are called___________________ __________. When atoms _________ together, they can make larger building blocks of matter called ____________.
2 Answer the following questions.
. How would you define chemistry?
. What was your first encounter with chemistry?
. What is/isn't interesting about chemistry for you?
. Which branch of chemistry would you like to specialize in?
. Name some branches of applied chemistry.
. Which sciences are closely connected to chemistry?
. Do you know any Nobel laureate in chemistry?
. Which skills should a chemist have?
. Where can you find a job as a chemist?
. Name some products which don´t exist without chemistry.
. What does organic/inorganic chemistry study?
3 Form the words and explain their meaning in English. Try to use all these words in
one sentence.
CHEM ___ ___ ___ ___ ___ (noun)
___ ___ ___ ___ (noun)
___ ___ ___ (noun)
___ ___ ___ ___ (adjective)
4 Match the definitions of the form of matter and then fill in the chart.
a) a system of two or more chemical substances which are not chemically bound
b) anything that occupies space and has mass
c) a substance formed by the combination of elements in fixed proportions to form a knew
substance by chemical reactions
d) a substance that cannot be decomposed into simpler substances by chemical reactions
e) a single pure form of matter
1. matter 2. substance 3. element 4. compound 5. mixture

5 Form collocations with an adjective chemical and use them in sentences.
The first one is done for you.
chemical a) a statement containing chemical symbols used to show the changes that
happen during a chemical reaction equation
b) the ways in which substance behaves in different circumstances
c) the process in which the structure of atoms and molecules that make up
a substance are changed
d) the way of representing a substance using the symbols of its elements
e) a scientific test done in order to discover if something works or is true
f) a general statement that is confirmed by observation
6 There are three basic states of matter below.
a) Describe them (fill in the gaps) using adjective definite and its antonym.
solids = ____________________ shape and volume
gases = ____________________ shape and volume
liquids = __________________ shape but ___________________________ volume
b) Name other phases.
c) Explain the difference between a chemical change and a physical change.
d) Form adjectives from the nouns solid, liquid, gas.
e) Form verbs from the nouns solid, liquid, gas.
f) Use one word to describe liquids and gases.
7 Complete the text, the first letters are given in bold.
The temperature at which a solid becomes a liquid is its m______________ p_________
and the point at which a liquid becomes a gas is its b _________________ p ________ .
When a gas or liquid becomes a solid, it s ______________ . When a gas or solid becomes
a liquid, it l __________________ . When a substance becomes a gas or v ____________,
it e ______________ or v ________________; if it returns to its previous state, it
c_____________.
8 Explain the following expressions:
1. irreversible reaction
2. a source of heat
3. trace amount
4. poisonous vapours
5. to be utilized by the body
9 Choose the correct alternative to complete the definitions.
1. a substance that increases the rate of a chemical reaction without itself being changed is a
a) inhibitor b) synthesiser c) catalyst
2. a liquid that dissolves substances is a
a) solvent b) solute c) solubility
3. a substance that allows heat or electricity to go through it is a
a) conductor b) detector c) condenser
4. a small amount of a substance that is used for scientific tests is a
a) trace b) sample c) drop
5. a liquid with another substance dissolved in it, so it has become part of the liquid is a
a) solution b) dilution c) desiccation
6. a liquid mixed with water or another liquid to make it less strong is
a) intact b) soluble c) dilute
10 Pronounce properly the following words. Check your knowledge of their meaning.
acid, acidic, alkali, aqueous, arrangement, atom, binary, Celsius, Centigrade, chemistry, chemical, density, to determine, to distinguish, equation, equilibrium, hormone, to ionize, isotope, nuclei, to occur, occurrence, pressure, procedure, pure, substance, surface, structure, technique, technology, vacuum, valency, to weigh
11 Fill in the missing prepositions.
1. Water boils _____ 100ฐ C.
2. You should lower the temperature ____ several degrees.
3. _______ these conditions the reaction doesn´t occur.
4. Which Czech scientists have been awarded Nobel Prize _____ chemistry?
5. Air is composed mainly _____ nitrogen and oxygen.
6. Chemical equilibrium may be classified _____ two groups.
7. What is capable _____ accepting a proton from another substance?
8. Solar power is the conversion of the sun´s energy ______ heat and electricity.
9. Neutrons are not electrically attracted ___ either electrons or protons.
10. I´m a chemist ____ profession.
11. I want to specialize____ biochemistry.
12. His thesis deals ______ nanomaterials.
12 Form adjectives from the nouns given below and use them in the following
sentences.
mole, science, molecule, density, atom, metal
1. To explore solids and liquids at the ____________ and ______________ level,
we need to look inside them.
2. Helium is a non-metal, although the ending ium is usually reserved for ญญญญญญญญญญญญญญญญญญญญญญญญญ _______________ elements.
3. Plutonium is very ____________________.
4. We need to adopt a more _______________ approach to this problem.
5. What is a ________________ weight of carbon?
13 Form antonyms.
abundant element ______________ effective method __________________
soluble salts ___________________ to absorb light ___________________
divisible particle ________________ volatile gas ______________________
practical research _______________ to increase temperature _____________
miscible with water _____________ pure substance_________________ญ___
stable equilibrium ______________ organic chemistry _________________
diluted solution _______________ to heat a substance _________________
similar properties _______________ thin layer ________________________
14 Put the stages in the scientific method in the correct order (number them 1-8).
a) Describe what the scientist must do using the connectors first/next/then/finally.
b) Form nouns from these eight verbs.
__ define the question __ collect/gather data
__ analyse data __ interpret data
__ draw conclusions __ conduct/carry out/perform an experiment
__ form a hypothesis __ design an experiment
15 Discuss acids, bases, alkalis.
a) Form the definition of an acid and a base using the following words:
accept, donate
b) Finish the equation for neutralization:
acid +_________ → ___________ + __________
c) What do the terms strong and weak acid refer to?
c) What is an alkali?
e) How do acids and bases change the colour of a litmus paper?
f) Form adjectives which mean containing acid, base, alkali.
a) Form nouns describing the quality of being acid, base, alkali.
16 Complete the information about oxidation and reduction.
a) Form the definition of oxidation and reduction using a mnemonic device OILRIG.
(oil rig = a large piece of equipment for getting oil from under sea)
oxidation is _________________________________
reduction is _________________________________
b) Form verbs and adjectives from the nouns oxidation and reduction.
c) Explain the terms oxidant and reductant.
d) What is a redox reduction?
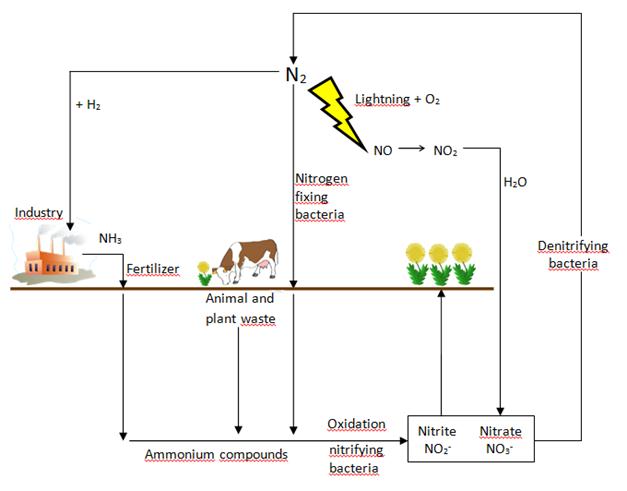
e) Nitrogen fixation is an example of a redox reaction in nature. Study the diagram and
explain the nitrogen cycle. Describe nitrogen fixation, the conversion of nitrogen,
assimilation, ammonification, nitrification and denitrification.
Date: 2015-01-12; view: 3364
| <== previous page | | | next page ==> |
| VOCABULARY AND GRAMMAR | | | Read the text about periodic table and then answer the questions. |