
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Neurocircuitry of the Brain Stress Systems in Dependence
Five potential arousal-stress neurotransmitter systems (CRF, norepinephrine, vasopressin, orexin, dynorphin) and two potential antistress neurotransmitter systems (NPY, nociceptin) have been explored in the present review from the perspective of a role in the neuroadaptation associated with the development of negative emotional states associated with drug dependence and addiction. The most compelling data are in the domain of CRF, where, for virtually all major drugs of abuse, (1) CRF is released during acute withdrawal, (2) CRF antagonists block the anxiogenic-like effects of acute withdrawal, (3) CRF antagonists block the excessive drug intake associated with dependence, and (4) CRF antagonists block stress-induced reinstatement. The focal point for most of these effects is the central nucleus of the amygdala and the bed nucleus of the stria terminalis (see Figure 1).
Although less extensive, similar data exist for some noradrenergic antagonists that block the anxiogenic-like effects of opiate withdrawal, block excessive drug intake associated with dependence on ethanol, cocaine, and opioids, and block stress-induced reinstatement to cocaine, opioids, ethanol, and nicotine (see Figure 4). Again, the focal point for many of these effects is the central nucleus of the amygdala and the bed nucleus of the stria terminalis.
Much evidence has been marshaled to show that dynorphin is increased in the nucleus accumbens in response to dopaminergic activation and, in turn, that overactivity of the dynorphin systems can decrease dopaminergic function. κ antagonists have been shown to block the aversive effects of drug withdrawal and the excessive drinking associated with ethanol dependence and stress-induced reinstatement of drug seeking (see Figure 5). Evidence suggests that κ receptor activation can produce CRF release (Song and Takemori, 1992), but recently some have argued that the effects of dynorphin in producing negative emotional states are mediated via activation of CRF systems (Land et al., 2008).
Much less evidence to date has demonstrated a direct role for vasopressin and orexin in the negative emotional states associated with drug dependence (see Figures 6 and and7).7). A vasopressin antagonist blocked stress-induced reinstatement of heroin-seeking behavior and withdrawal-induced ethanol drinking, and an orexin antagonist blocked stress-induced reinstatement of cocaine seeking. Much more work will be required to explore the role of these systems and their interactions with other major players, such as CRF.
Significant evidence suggests that activation of NPY in the central nucleus of the amygdala can block the motivational aspects of dependence associated with chronic ethanol administration. NPY administered intracerebroventricularly blocked the anxiogenic-like effects of withdrawal from ethanol and blocked the increased drug intake associated with ethanol dependence (see Figure 8). Direct administration or viral vector-enhanced expression of NPY into the central nucleus of the amygdala also blocked the increased drug intake associated with ethanol dependence. Few or no studies have examined the effects of NPY on motivational aspects of dependence with other drugs of abuse.
The role for nociceptin in dependence suggests interactions both with the rewarding effects of drugs of abuse and in the motivational aspects of dependence, mainly with ethanol. Nociceptin blocks the rewarding effects of most major drugs of abuse measured by place preference (see Supplemental Data). Nociceptin decreased ethanol self-administration in msP rats known to have a constitutive increase in CRF activity and a stress-like phenotype. msP rats are known to have a high basal stress response, to show decreased ethanol intake similar to dependent rats with administration of a CRF1 antagonist, and to carry a genetic polymorphism of the CRF1 promoter, resulting in increased CRF1 density in several brain regions (Hansson et al., 2006) (see Figure 9). Nociceptin also significantly reduced stress-induced reinstatement of ethanol. Future studies should explore the role of both of these antistress systems (NPY, nociceptin) in the negative emotional responses associated with dependence on other drugs of abuse.
A pronounced interaction exists between central nervous system CRF and norepinephrine systems. Conceptualized as a feed-forward system at multiple levels of the pons and basal forebrain, CRF activates norepinephrine, and norepinephrine in turn activates CRF (Koob, 1999; see Supplemental Data).
The common neurocircuitry actions of drugs of abuse on the brain stress systems and the change in plasticity of these circuits (see above) may involve molecular neuroadaptations that either differentially drive the circuits or result from the changes in activity of the circuits or both. Repeated perturbation of intracellular signal transduction pathways may cause changes in neuronal function and/or changes in nuclear function and altered rates of transcription of particular target genes. Altered expression of such genes would lead to presumably long-term altered activity of the neurons where such changes occur and ultimately to changes in neural circuits in which those neurons operate. Much work in addiction has shown that chronic exposure to opiods and cocaine leads to activation of CREB in the nucleus accumbens and central nucleus of the amygdala (Shaw-Lutchman et al., 2002; Edwards et al., 2007). Although acute administration of drugs of abuse can cause a rapid (within hours) activation of members of the Fos protein family, such as FosB, Fra-1, and Fra-2 in the nucleus accumbens, other transcription factors, isoforms of ΔFosB, have been shown to accumulate over longer periods of time (days) with repeated drug administration (Nestler, 2005). Animals with activated ΔFosB have exaggerated sensitivity to the rewarding effects of drugs of abuse, and ΔFosB may be a sustained molecular ?switch? that helps to initiate and maintain a state of addiction (McClung et al., 2004). Whether (and how) such transcription factors influence the function of the brain stress systems, such as CRF and those described above, remains to be determined.
A focus of this review has been on the connections of the brain arousal-stress systems with the extended amygdala, particularly the central nucleus of the amygdala and the bed nucleus of the stria terminalis. Three of the seven systems (norepinephrine, orexin, NPY) are widely distributed in the brain but with a heavy innervation of the extended amygdala. Four of the systems (CRF, vasopressin, nociceptin, dynorphin) are more localized to local circuits throughout the forebrain but also with a heavy innervation of the extended amygdala (Figure 10). However, the convergence of these neurotransmitter systems in the region of the extended amygdala suggests key roles in the processing of emotional stimuli potentially triggered by neurons deriving from the brainstem (norepinephrine), hypothalamus (nociceptin, NPY), and within the extended amygdala itself (CRF, vasopressin, nociceptin, dynorphin). The extended amygdala receives afferents from the prefrontal cortex and insula and sends efferents to the lateral hypothalamus, ventral tegmental area, and pedunculopontine nucleus (Figure 10). Which parts of this neurocircuitry play a key role in the negative emotional states of drug dependence and how they interact with the brain stress systems remain to be elucidated. What is known is that most of the cells in the lateral division of the central nucleus of the amygdala and bed nucleus of the stria terminalis (extended amygdala) are GABAergic and that a distinct subpopulation colocalizes with either enkephalin or CRF, but they virtually never colocalize together on the same GABAergic cell (Day et al., 1999). Only enkephalin, and not CRF, colabeled neurons were activated by interleukin-1β, suggesting that discrete neural circuits exist within the extended amygdala (Day et al., 1999). Additionally, the electrophysiological anatomical studies outlined above suggest that these GABAergic neurons in the central nucleus of the amygdala respond to arousal-stress neurotransmitters with increased firing and respond to antistress neurotransmitters with decreased firing. These GABAergic neurons that are intrinsic to the central nucleus of the amygdala may be interneurons that inhibit another GABAergic link in the efferent pathway (Day et al., 1999; Davis et al., 1994).
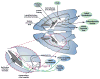
Figure 10
Date: 2016-06-12; view: 640
| <== previous page | | | next page ==> |
| Cellular Mechanisms of the Brain Stress Systems in the Extended Amygdala | | | Opponent Process/Between-System Neuroadaptations |