
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Fluid responsiveness and the haemodynamic effects of fluids in patients with sepsis
Studies in heterogeneous groups of critically ill and injured pa- tients and those undergoing surgery have reproducibly demon- strated that only about 50% of haemodynamically unstable patients are fluid responders.5054–56This is a fundamental con- cept which is not widely appreciated,5758and challenges the widely accepted notion that fluid administration is the ‘corner- stone of resuscitation’.5–759As a result of the effects of sepsis on the venous capacitance vessels and myocardial function, it is likely that less than 40% of hypotensive patients with severe sepsis or septic shock are ‘fluid responders’.60–62
The goal of fluid resuscitation is to increase the stressed blood volume and MCFP more than the CVP, and thereby increase the pressure gradient for venous return. However the ability of crys- talloids (the most common fluid used for the resuscitation of patients with sepsis) to expand the intravascular volume is
poor. Chowdhury and colleagues63 reported that in healthy
volunteers, only 15% of a crystalloid bolus remained in the intra- vascular space at 3 h, with 50% of the infused volume being in the
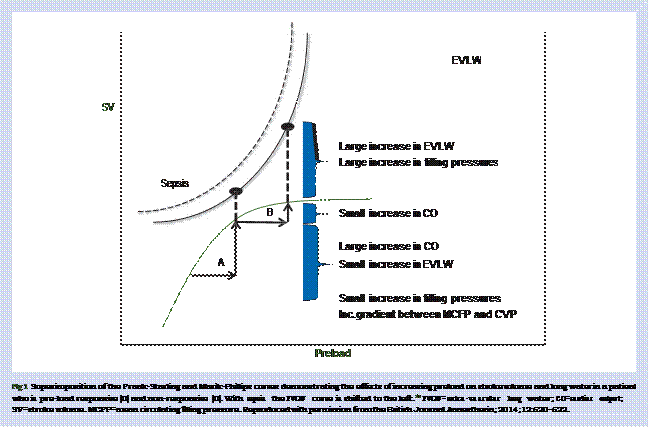
extravascular extracellular compartment. In patients with sepsis and in experimental models, less than 5% of a crystalloid bolus remains intravascular an hour after the end of the infusion.6465It is therefore likely that the haemodynamic effects of a fluid bolus (in the fluid responders) are short-lived, with the net effect
being the shift of fluid into the interstitial compartment with tis- sue oedema. Nunes and colleagues66demonstrated that in fluid responders, the SV returned to baseline 60 min after a crystalloid bolus. Glassford and colleagues67performed a systematic review which examined the haemodynamic response of fluid boluses in patients with sepsis. These authors reported that while the mean arterial pressure (MAP) increased by 7.8 (3.8) mm Hg immediately after the fluid bolus, the MAP had returned close to baseline at one h with no increase in urine output. In a retrospective analysis of the ARDSnet Fluid and Catheter Treatment Trial (FACTT),68Lammi and colleagues62 examined the physiological effect of 569 fluid boluses (15 ml kg−1; 1025±243 ml) in 127 patients (the majority of whom were septic), randomized to the pulmonary ar- tery catheter arm of the study. The FACTT trial required reassess- ment of the haemodynamic profile one h after the fluid bolus, if the indication for fluids was shock, ineffective circulation, or low urine output and four h if the indication was a low pulmonary artery occlusion pressure (PAOP).68Fifty-eight percent of fluid boluses were given for shock or poor urine output/ineffective cir- culation, with 42% of boluses given for a low PAOP. In this study, only 23% of patients were fluid responders (increase in CI > 15%).
There was a small increase in the MAP (78.3 16.4 to 80.4 16.5 mm Hg) while the urine output did not change in the 1–4 h after the fluid bolus.
Monge-Garcia and colleagues69 measured the effects of a
fluid bolus on arterial load in patients with septic shock. In this study 67% of patients were fluid responders, however the MAP increased in only 44% of these patients ( pressure responder). Over- all there was a significant reduction in effective arterial elastance (Ea) and systemic vascular resistance (SVR), this effect being most marked in the pre-load responders who were pressure non- responders. Additional studies have demonstrated a decrease in
SVR after fluid resuscitation in patients with sepsis.7071This sug-
gests that fluid boluses should be considered vasodilator therapy, in patients with sepsis and that aggressive fluid resuscitation may potentiate the hyperdynamic state.
In summary, these studies demonstrate that the majority of patients with severe sepsis and septic shock are not fluid respon- ders. Furthermore, the haemodynamic changes in the fluid re- sponders are small, short-lived and likely to be clinically insignificant. However, aggressive fluid resuscitation will likely have adverse haemodynamic consequences including an in- crease in cardiac filling pressures, damage to the endothelial gly- cocalyx, arterial vasodilation and tissue oedema. Consequently, the concept that aggressive fluid resuscitation is the ‘cornerstone
of resuscitation’ of patients with severe sepsis and septic shock needs to be reconsidered.5–759Indeed, it is likely that aggressive fluid resuscitation increases the morbidly and mortality of pa- tients with sepsis (see section below). Nevertheless the updated Surviving Sepsis Campaign Guidelines, published after the publi- cation of the ProCESS, ARISE and PROMISE studies8–10mandate the administration of ‘30 ml kg−1 crystalloid for hypotension or lactate ≥4 mmol Litre-1’ within 3 h of presentation to hospital.72This recommendation is problematic as the majority of hypoten- sive patients with septic shock will not respond to fluids; this ap- proach is likely to lead to ‘salt water drowning’ with an increase in the morbidity and mortality of these patients.73Furthermore, as discussed below, an increased blood lactate is unlikely to be associated with anaerobic metabolism, or inadequate oxygen
delivery, and attempts at increasing oxygen delivery do not in- crease oxygen consumption or lower lactate concentrations. In- deed such an approach has been demonstrated to increase the risk of death of critically ill patients.74
These data suggest that only patients who are fluid respon- sive should be treated with fluid boluses. Furthermore, the patients’ fluid responsiveness and the risk/benefit ratio of fluid administration needs to be determined before each fluid bolus.75As the haemodynamic response to a fluid challenge is very short-lived and large fluid boluses (20–30 ml kg−1) are associated with severe volume overload, the mini-fluid bolus approach (200–500 ml) to fluid therapy is recommended.76The passive leg raising manoeuvre (PLR) and the fluid bolus test coupled with real-time SV monitoring, are currently the only techniques which have an acceptable degree of clinical accuracy, which can be used for determining fluid responsiveness.51Because of its ease of use, simplicity, high diagnostic accuracy, inherent safety and short procedure time (less than 5 min to per- form) the PLR is the preferred method to assess fluid responsive- ness in the emergency department, hospital ward and ICU.5175The PLR manoeuvre is performed by lifting the legs passively from the horizontal position and is associated with the gravitational transfer of blood (about 300 ml) from the lower limbs and abdo- men toward the intrathoracic compartment.757778The PLR man- oeuvre has the advantage of reversing its effects once the legs are returned to the horizontal position.757980Therefore, the PLR manoeuvre is considered a reversible or ‘virtual’ fluid challenge. The ability of the PLR manoeuvre to serve as a test of preload re- sponsiveness has been confirmed in multiple studies performed in critically ill patients. A meta-analysis, which pooled the results of eight studies, confirmed the excellent value of PLR to predict fluid responsiveness in critically ill patients with a global area under the ROC curve of 0.95 (95% CI, 0.92–0.95).81In an updated meta-analysis which evaluated 21 studies, we report a pooled ROC AUC of 0.93–0.95 (Monnet X, Marik P, Teboul JL; submitted for publication). As the maximal haemodynamic effects of PLR occur within the first min of leg elevation,7580it is important to assess these effects with a method able to track changes in car- diac output or SV on a real-time basis. It is important to note that the change in bp after a PLR or fluid challenge is a poor guide to fluid responsiveness; SV may increase without a signifi- cant change in bp.70Furthermore, unlike techniques to deter- mine fluid responsiveness based on heart-lung interactions, the PLR manoeuvre can be performed in spontaneously breathing patients, patients with cardiac arrhythmias and those receiving low tidal volume ventilation.7551
The chest radiograph, CVP, central venous oxygen saturation (ScvO2) and ultrasonography, including the vena-caval collaps- ibility index, have limited value in guiding fluid management and should not be used for this purpose.5482–86Furthermore, it has been well established that physical examination cannot be used to predict fluid responsiveness and physical examination is unreliable for estimating intravascular volume status.87It is therefore very troubling that the updated Surviving Sepsis Campaign Guidelines which are now federally mandated in the USA (SEP-1 Early Management Bundle, #0500 Severe Sepsis and Septic Shock: management Bundle) require either a ‘focused exam by a licensed independent practitioner’, or measurement of the CVP or ScvO2, or bedside cardiovascular ultrasound, to assess the volume status of the patient with severe sepsis and septic shock.88It should be noted that the area under the receiver oper- ator characteristic (ROC) curve of the CVP, for predicting fluid re- sponsive is approximately 0.5, which is considered a ‘completely useless test’.54 89 90 Furthermore, it is important to emphasize
that a normal CVP is between 0–2 mm Hg; this is necessary to en- sure adequate venous return and cardiac output (as discussed above). In addition, while the change in CVP in response to a fluid challenge is still widely promoted as a method to guide fluid therapy,57this technique has no physiologic basis and is unable to predict fluid responsiveness with any degree of accur- acy.5491Furthermore, it should be noted that with the exception of measuring dynamic changes in the carotid Doppler peak velocity,869293bedside ultrasound including the inferior vena caval distensibility index cannot accurately predict fluid respon- siveness.51828586It is somewhat astonishing that the ScvO2 is still being recommended to guide the resuscitation of critically ill septic patients and is being used as an indicator of the quality of care delivered.7288Monitoring the ScvO2 in patients with sepsis has no scientific basis, as patients with sepsis usually
have a normal or increased ScvO2,9495and a high (ScvO2 > 90%)
sepsis is considered to be a ‘hypermetabolic’ condition oxygen consumption and energy expenditure are broadly comparable with that of normal people, with energy expenditure decreasing with increasing sepsis severity.103–105Therefore, there is no re- quirement that oxygen delivery increase with sepsis. Indeed, in-
creasing oxygen delivery in patients with sepsis does not increase oxygen consumption nor decrease lactate concentra- tions.106107The critical oxygen delivery threshold for humans (both septic and non-septic) is approximately 3.8 (1.5) ml min−1
kg−1 (270 ml min−1 in a 70 kg patient).108These values translate into a cardiac output of approximately 2 Litre min−1; it is likely that only pre-terminal moribund patients with septic shock would have such a low cardiac output.
Date: 2016-04-22; view: 795
| <== previous page | | | next page ==> |
| Pertinent normal cardiovascular physiology | | | Evidence supporting the deleterious effects |