
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Detoxifying liver function
Detoxification of substances in the liver is their chemical modification, which usually involves two phases. In the first phase a substance undergoes oxidation, reduction, hydrolysis, resulting in the formation of the -OH, -COOH, -SH, -NÍ2, and other groups. In the second phase some substance - glucuronic acid, sulfuric acid, glycine, glutamine, acyl residue is joined to this groups (conjugation reactions). In some cases, detoxification involves only one phase - the first or the second. Some substances are partially or completely removed without any change at all. The main role in the reactions of the first phase of detoxification belongs to microsomal enzymes (monooxygenases). The main component of the microsomal oxidation system is cytochrome P450. In the endoplasmic reticulum of hepatocytes, there are many isoforms of P450, all of which are characterized by broad substrate specificity, yet differ in specificity. They can catalyze not only hydroxylation, but also the reactions of other types. NADP • H and molecular oxygen are used in reactions.
 Hydroxylation: R–Í® R–ÎÍ
Hydroxylation: R–Í® R–ÎÍ
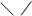 Epoxidation: R–CÍ=ÑÍ–R' ® R–CH–CH–R'
Epoxidation: R–CÍ=ÑÍ–R' ® R–CH–CH–R'
O
 Sulfoxidation: R–S–R' ® R–S–R'
Sulfoxidation: R–S–R' ® R–S–R'
Î
Dealkylation: RÎÑÍ3® RÎÍ + Í2Ñ=Î
RNHÑÍ3® RNH2 + Í2Ñ=Î
Reduction of nitro compounds: RNÎ2® RNH2
An example of the reactions of the first phase neutralization is the hydroxylation of benzene:
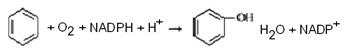
benzene phenol
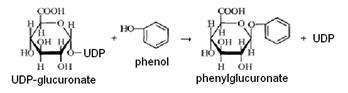
The most common reaction of conjugation is glucuronic acid binding with the glucuronide formation. Glucuronic acid donor is UDP-glucuronate (UDPG); the reaction is catalyzed by glucuronyl transferase - an integral protein of the endoplasmic reticulum:
In the reaction of conjugation with sulfuric acid, sulfuric acid residue donor is 3'-5-phosphoadenosine-5-phosphosulfate (PAPS, PAP-SO3Í):
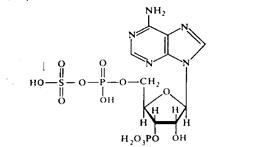
The formation of conjugate with phenol (phenyl sulphate) is as follows:
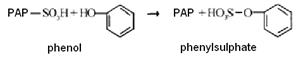
In the reactions of oxidation and conjugation hydrophilic groups are formed in molecules of neutralized substances, the substance becomes more soluble in water, which facilitates its excreation from the body. In addition, chemical modification of toxic substances, tend to reduce their toxicity.
As a result of putrefactive processes occurring in the intestine, phenol and cresol are formed from tyrosine, and skatole and indole from tryptophan. These substances are absorbed and enter the liver with the bloodstream, where they are detoxified by the formation of compounds with sulfuric or glucuronic acid or, more precisely, with their active forms: PAPS and UDPG.
Aspirin (acetylsalicylic acid) is widely used as an anti-inflammatory drug. It is excreted after conjugation with glucuronic acid or glycine, as well as in the form of gentisic acid. As a result of these biochemical reactions the inactivation of drugs (e.g. benzodiazepines) usually occurs. However, some metabolites are active (cortisone metabolite cortisol, prednisone metabolite prednisolone).
Some substances (barbiturates, haloperidol) induce microsomal liver enzymes, especially cytochrome P450; other substances (chloramphenicol, allopurinol) inhibit them. Ethanol may have both effects. Simultaneous treatment of two drugs metabolized by the same microsomal enzymes, can lead to increase or decrease of the pharmacological action of one or both.
Decreased activity of microsomal enzymes can result in slower inactivation and elimination of certain drugs (phenobarbital, glucocorticoids, tetracyclines, trimethoprim) and many others. This leads to a decrease in therapeutic dose and therapeutic range.
The mechanisms responsible for the elimination of drugs can lead to the formation of hepatotoxic compounds. For example, the metabolism of acetaminophen (paracetamol) by liver enzymes leads to the formation of a high active free radical that can irreversibly inactivate many enzymes and other proteins by binding to their sulphhydryl groups. Normally, the toxic effect of this radical is prevented by the reaction with glutathione. However, at overdose of paracetamol or damage of the liver glutathione in hepatocytes is rapidly used. It can lead to inactivation of cellular proteins and extensive necrosis of hepatocytes. If there is paracetamol overdose you should quickly inject the N-acetylcysteine (NAC) - the substance rich in sulfhydryl groups.
Test Questions
1. Describe the chemical composition of the liver.
2. What role does the liver play in carbohydrate and lipid metabolism?
3. What reactions of metabolism of amino acids and proteins occur in the liver?
4. What is the detoxifying function of the liver?
5. What is the mechanism of neutralization of xenobiotics in the liver?
6. What is the role of the liver in the metabolism of bilirubin?
7. What is the direct and indirect bilirubin?
8. What types of jaundice you know? What are their and symptoms?
9. What tests will distinguish obstructive jaundice from hemolytic jaundice?
Date: 2016-04-22; view: 1507
| <== previous page | | | next page ==> |
| Choleresis. Pigment metabolism. Types of jaundice | | | PHOSPHORUS METABOLISM |