
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
Results and Discussion
3.1. In Vitro Growth Inhibition
The rich MRS medium was chosen for these experiments because it supported vigorous growth by theLactobacillus, Staphylococcus aureus, and C. albicans strains. Agar overlays of C. albicans on MRS plates inoculated with the probiotic lactobacilli and control bacteria represented a relatively simple screening method for growth inhibitory activities of the bacteria towards this yeast. The deferred assay regimen with a 2-day preincubation of the bacteria was the method of choice because simultaneous inoculation often led to no or very small bacterial colonies due to rapid overgrowth by the fungal cells.
After 48  hrs of incubation, the probiotic Lactobacillus—C. albicans overlays revealed clearly visible zones of fungal growth inhibition around the Lactobacillus GR-1 and RC-14 colonies (see Figure 1(a)) on MRS plates. Very weak or no inhibition zones were found around the colonies of the control strains L. johnsoniiPV016 and S. aureus ATCC 25923, respectively. PV016 was isolated by us from the prairie vole intestine and classified by molecular methods as L. johnsonii (data not shown). This strain appears to have only limited anti-Candida activity. Despite the vigorous growth of the S. aureus strain on MRS, no antifungal activity was detected in the overlay assay. S. aureus is not considered a lactic acid bacterium and appears to produce only limited amounts of lactic acid under aerobic conditions [15]. On buffered MRS-MOPS agar plates the clear areas around the probiotic lactobacilli were much smaller or absent. Thus, the inhibitory activities of lactobacilli towards C. albicans appear to be decreased when the media is buffered with MOPS to an initial pH of 7.0 before inoculation. The pH of unbuffered MRS media was 6.5 before inoculation. The formation of lactic acid and other organic acids by the growing probiotic lactobacilli is likely to cause a substantial decrease in the pH of unbuffered MRS.
hrs of incubation, the probiotic Lactobacillus—C. albicans overlays revealed clearly visible zones of fungal growth inhibition around the Lactobacillus GR-1 and RC-14 colonies (see Figure 1(a)) on MRS plates. Very weak or no inhibition zones were found around the colonies of the control strains L. johnsoniiPV016 and S. aureus ATCC 25923, respectively. PV016 was isolated by us from the prairie vole intestine and classified by molecular methods as L. johnsonii (data not shown). This strain appears to have only limited anti-Candida activity. Despite the vigorous growth of the S. aureus strain on MRS, no antifungal activity was detected in the overlay assay. S. aureus is not considered a lactic acid bacterium and appears to produce only limited amounts of lactic acid under aerobic conditions [15]. On buffered MRS-MOPS agar plates the clear areas around the probiotic lactobacilli were much smaller or absent. Thus, the inhibitory activities of lactobacilli towards C. albicans appear to be decreased when the media is buffered with MOPS to an initial pH of 7.0 before inoculation. The pH of unbuffered MRS media was 6.5 before inoculation. The formation of lactic acid and other organic acids by the growing probiotic lactobacilli is likely to cause a substantial decrease in the pH of unbuffered MRS.

Figure 1
Agar inhibition assays with bacteria, lactic acid, and C. albicans. The images in (a) show the results of deferred agar overlay assays on bacterial colonies (L. rhamnosus GR-1, L. reuteri RC-14, L. johnsoniiPV016, and S. aureus ATCC 25923) and C. albicans ...
In comparison to the probiotics, the L. johnsonii and S. aureus control strains apparently were not able to produce sufficient acid or other metabolic products to inhibit the fungi. On the other hand, the control results suggest that potential glucose or other nutrient exhaustion around the bacterial colonies is not a likely cause for fungal growth inhibition in the overlay assay. C. albicans also produces acids during aerobic growth and can drastically reduce the pH of batch cultures in some media [16, 17]. However, in an overnight culture of C. albicans grown in MRS at 37°C, we observed a pH reduction to only pH 6.0. In a disk diffusion assay with lactic acid, the potential inhibition of C. albicans growth by the acid was confirmed (Figure 1(b)). Clear zones around the disks were visible if the concentration of the acid was sufficiently high. However, performing the disk diffusion assay on MRS-MOPS revealed that buffering diminished the antifungal activity, similar to the overlay assays with bacteria.
For a more detailed assessment of the acid tolerance of C. albicans in MRS broth, we conducted growth assays with MRS containing lactic acid at different pH levels (not pH adjusted or adjusted to pH 7.0 and 4.5), and MRS adjusted to pH 4.5 using hydrochloric acid (Figure 2). These experiments revealed that C. albicans SC5314 growth is decreased at lower pH levels. The strongest growth inhibition was detected in MRS that contained lactic acid (120  mM) with no pH adjustment. Addition of 120
mM) with no pH adjustment. Addition of 120  mM lactic acid to MRS resulted in an actual pH of 4.2. The growth inhibitory effect of this high concentration of lactic acid was reduced by pH adjustment to 4.5 and completely abrogated by neutralization to pH 7.0 with sodium hydroxide. Adjustment of the pH of MRS to 4.5 using hydrochloric acid led to intermediate growth reduction which was significantly lower than the reduction achieved by 120
mM lactic acid to MRS resulted in an actual pH of 4.2. The growth inhibitory effect of this high concentration of lactic acid was reduced by pH adjustment to 4.5 and completely abrogated by neutralization to pH 7.0 with sodium hydroxide. Adjustment of the pH of MRS to 4.5 using hydrochloric acid led to intermediate growth reduction which was significantly lower than the reduction achieved by 120  mM lactic acid at pH 4.5. These results indicate that C. albicans appears to be sensitive to low pH and lactic acid.
mM lactic acid at pH 4.5. These results indicate that C. albicans appears to be sensitive to low pH and lactic acid.

Figure 2
Inhibition of C. albicans growth by lactic acid at low pH. Microplate growth assays were used to determine the effect of lactic acid on the growth of C. albicans. MRS medium was supplemented with 120  mM lactic acid and the pH was adjusted to pH ...
mM lactic acid and the pH was adjusted to pH ...
In conjunction with acid stress, the increased concentration of membrane-permeable, undissociated lactic acid (pKa = 3.86) at low pH could have an inhibitory effect on the growth of the pathogenic yeast. Undissociated lactic acid permeates the plasma membrane by diffusion and subsequently dissociates into protons (H+) and lactic acid counterions. The charged ions are unable to cross the membrane bilayer, accumulate, and interfere with cell metabolism by acidification of the cytosol. Inhibition of fungal growth through lactic acid production by lactobacilli might therefore be most efficient at low pH.
Growth inhibition assays using culture filtrates from L. rhamnosus GR-1 and L. reuteri RC-14 indeed revealed that C. albicans growth was suppressed at low pH by the bacterial culture supernatants. Neutralization of the culture filtrates, however, completely abrogated the inhibitory effects of these supernatants (Figure 3). While the contribution of additional antifungal compounds produced by the lactobacilli in inhibiting C. albicans is unknown at present, our results indicate that lactic acid at low pH plays a major role in keeping fungal growth in check. Hydrogen peroxide production by L. reuteri RC-14 could be an additional anti-Candida factor. In contrast to L. rhamnosus GR-1, L. reuteri RC-14 produces H2O2 constitutively on MRS (data not shown). However, the relatively high resistance of C. albicans to this potential growth inhibitor likely diminishes its efficacy. The growth assays conducted in this study did not show that L. reuteri RC-14 was more potent in inhibiting C. albicans, despite the strain's ability to generate H2O2.
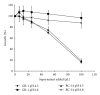
Figure 3
Growth inhibition of C. albicans by culture supernatants from probiotic lactobacilli. Cell-free culture supernatants of L. rhamnosusGR-1 and L. reuteri RC-14 were collected after 48  hrs of growth and adjusted to pH 4.5 and pH 6.8 using sodium ...
hrs of growth and adjusted to pH 4.5 and pH 6.8 using sodium ...
Date: 2016-01-03; view: 1037
| <== previous page | | | next page ==> |
| Transcriptional Profiling | | | Fungicidal Activities of Lactobacilli |