
CATEGORIES:
BiologyChemistryConstructionCultureEcologyEconomyElectronicsFinanceGeographyHistoryInformaticsLawMathematicsMechanicsMedicineOtherPedagogyPhilosophyPhysicsPolicyPsychologySociologySportTourism
The dynamics of ecosystems
The dynamics of ecosystems involves energy flow and chemical recycling. Two key ingredients are essential in the maintenance of any ecosystem Ė energy and inorganic elements. Letís think about where they come from. Most of the energy that enters an ecosystem is solar energy. This light energy is converted to chemical energy by photosynthetic organisms, also thought of as primary producers in an ecosystem. Energy may also enter the food chain in the form of chemical energy. For example in deep sea thermal vent communities primary producers derive energy by oxidizing compounds such as hydrogen sulfide and then use that energy to convert inorganic elements into organic compounds. In either case energy is then passed on to the consumers in the form of organic molecules or is lost as
heat. Since the energy entering an ecosystem is ultimately lost as heat ecologists consider energy to flow through an ecosystem rather than be recycled in it.
On the other hand, inorganic elements are persistent in an ecosystem in one form or another. They move through the ecosystem in biogeochemical cycles. These inorganic elements may be locked in an organism as part of organic molecules, or be present in the atmosphere or in rocks. The inorganic elements must be released into the ecosystem where producers can once again incorporate them into organic molecules. Ecologists therefore consider inorganic elements to be recycled in an
ecosystems. Detritivores (fungi and microorganisms) involved in the ecomposition of organic matter, connect all levels in the ecosystem.Both energy and inorganic elements move by the same pathway through an ecosystem, primarily via
photosynthesis, feeding and decomposition. Examine the figure shown here and note the different ecosystems listed on the left hand side. Also note that on a global scale net primary production, which represents the stored chemical energy available to consumers, varies with the different ecosystems. The oceans and tropical rain forest are the two largest contributors to the overall percentage of Earthís net primary production. In the ocean the net primary production per unit area is much lower than most of the other ecosystems, however the area covered by ocean is so vast that overall net primary production is large. The opposite is true of algal beds and coral reefs. These organisms have high primary production per unit area but overall they cover a small percentage of the Earthís surface.

The figure shown here is representative of the net primary production for Earth based on data such as chlorophyll density that can now be collected by satellite. One striking result is how unproductive most of the oceans are per unit area compared to tropical rainforest. Another striking result is that the oceans are not uniformly productive. Now letís think about why that occurs. The equatorial regions of the planet receive the greatest intensity of light so one might expect them to have the highest productivity. However, when we study the figure shown here we note that some of the more productive oceans are away from the tropical belt so something other than light must be limiting primary production. Nutrient enrichment experiments have shown that the availability of inorganic nutrients is more limiting to primary production in the ocean than light. The nutrient that is
most limiting in the photic zone is usually nitrogen or phosphorus. However in some areas when both nitrogen and phosphorus are plentiful primary production is still limited. For example in the Sargasso Sea, iron, a micronutrient was found to be the limiting factor for primary production. Experiments have shown that iron is required by cyanobacteria to convert atmospheric nitrogen into nitrogenous compounds that can be used by phytoplankton for growth. At this point you should be thinking to yourself, but why are some areas of the ocean low in iron. To answer this question you have to think back to the wind patterns around the globe. Many of the micronutrients that are delivered to the ocean are blown from the land masses as dust. Some areas of ocean, such as the Sargasso Sea receive relatively little dust.Areas of ocean with high primary productivity are usually in the vicinity of upwellings, where nutrients from the deeper water are circulated up to the photic
zone. These regions of high primary production are able to support larger fish
populations. Now letís give some consideration to terrestrial habitats and think about what limits primary productivity there. When we study the relative rates of primary production of the land masses above, we note that tropical forests are the main primary producer. Desert regions, such as those found in North Africa or the semi frozen tundra to the far north are low primary producers. So what is limiting primary production in terrestrial areas? If you said temperature and moisture are the most limiting factors to primary production in terrestrial ecosystems you would be correct. The most productive areas have warm temperatures and moisture all year long optimizing plant growth. Nutrient availability also affects plant growth but tends to be more localized. As in marine ecosystems, nitrogen is the limiting element for terrestrial plants since it is required in large amounts for normal growth and cell function.

During primary production, autotrophs convert solar energy to chemical energy
which is then locked inside the plant as plant biomass increases. In any ecosystem,
consumers eat the plant material that was produced as part of the food chain. The
chemical energy from the plant material is converted into new biomass in the
consumer. This phenomenon is called secondary production. Letís consider a
herbivore such as this caterpillar for our primary consumer. We know that
caterpillars eat a lot of plant material, but everything that is eaten is not converted to
new growth. Undigested plant material is excreted in feces; the energy it contains
remains in the ecosystem where it is digested by detritivores. The caterpillar
continuously undergoes cellular respiration which consumes a good deal of energy
most of which is lost from the ecosystem as heat. Whatever energy is left over
contributing to growth of the organism is known as the production efficiency. In this
case the production efficiency is 33/200 joules of energy or about 17% of food
consumed is converted to new biomass.
Birds and mammals have low production efficiencies in the range of 1 Ė 3%; fish
have a production efficiency around 10%. Insects are more efficient at converting
food to biomass with production efficiencies around 40%.

Now letís think about trophic efficiency or energy transfer from one trophic level to the next in a food chain. In general, trophic efficiencies range from 5 Ė 20%
depending on the ecosystem and this loss is multiplied through the food chain. The
figure above represents an idealized pyramid of net production with a trophic
efficiency of 10% for each link in the food chain. With a transfer of 10% of the
energy consumed being passed to the next level, then only 10/10,000J (0.1%) of
the net primary production is available to the tertiary consumer. The top consumer
in an ecosystem is typically a larger organism and will need to consume many prey
organisms in order to acquire enough energy to survive. The ecosystem shown on
this slide will support relatively few snakes compared to the number of field voles.
Now that you have the concept of energy flow through an ecosystem, compare how humans can impact the dynamics of energy flow through an ecosystem if they
consume their energy exclusively as primary consumers or secondary consumers.

The figure above shows a general model of nutrient cycling indicating the main
reservoirs of elements and how they are transferred in an ecosystem. Each
reservoir is characterized by two features: whether organic or inorganic molecules
are present and if they are available to an organism. Note that nutrients may be
locked up as biotic or abiotic components. The route a particular element takes
through a biogeochemical cycle will depend on the element and the trophic structure of an ecosystem. For example letís consider carbon. Carbon is converted to organic molecules via photosynthesis and is consumed in the food chain so some
carbon becomes locked up in the growing organism. Feces and dead organic
material are broken down into inorganic elements by decomposers releasing carbon molecules. Both plants and animals undergo cellular respiration and release carbon dioxide back into the atmosphere. This would be the local movement of carbon between reservoirs Ďaí and Ďcí. Letís say this carbon exchange occurred during the early Carboniferous period in the great swamps of that time. Some of the vegetation and dinosaurs became fossilized in sedimentary rock, others were
compressed into crude oil. Those particular carbon molecules have moved from
reservoir Ďaí to reservoirs Ďbí and Ďdí where they remain unavailable until some of
them are released back into the atmosphere by the burning of fossil fuels. Those
molecules that are locked up in rock are released back into the ecosystem as the
rock is weathered. Tracing elements through a particular biogeochemical cycle is
really not this straightforward since elements are exchanged between ecosystems
but a general pattern of movement can be established.
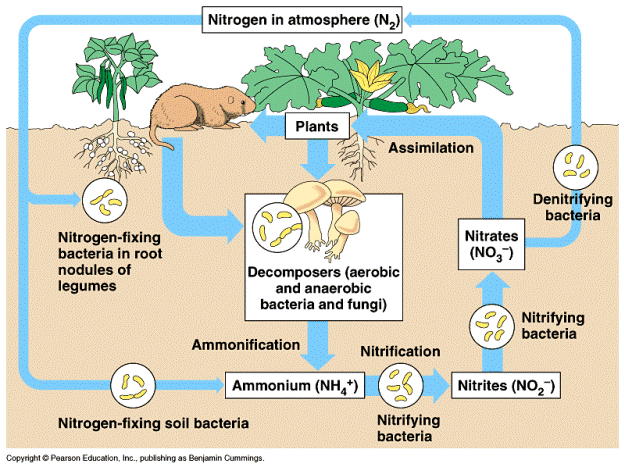
This figure shows a simple representation of nitrogen movement in an ecosystem.
The largest reservoir for nitrogen is the atmosphere which contains 80% nitrogen.
However, plants are unable to convert this nitrogen gas into organic molecules.
Nitrogen fixing bacteria in the soil may be free living or have formed an association with the roots of leguminous plants. These bacteria utilize the chemical energy in the triple nitrogen bonds producing ammonium ions in the process. Ammonium ions are also produced, as fungi and other microorganisms decompose organic material such as feces and dead plant and animal material, in a process called ammonification. Nitrifying bacteria in the soil convert the ammonium ions into nitrate ions in a process called nitrification. Nitrate ions are more readily absorbed by plants than ammonium ions. In wetland areas denitrifying bacteria use nitrate molecules for energy and in the process release nitrogen back into the atmosphere. In plants, nitrate and ammonium ions are assimilated into amino acids and proteins which in turn are consumed by animals. The strong triple bond of atmospheric nitrogen can also be broken by natural processes such as lightening or by artificial means during fertilizer production.
Under natural conditions nutrients are recycled continuously within an ecosystem.
However biogeochemical cycles are often disrupted by human activities. For
example, cultivation of land to grow annual crops such as wheat disrupts the
nutrient balance in the soil. As the wheat grows nutrients from the soil are locked
into the growing plant. At harvest time many of the nutrients are removed from the
area as the grain is shipped off for food and the straw is removed for animal
bedding or some other purpose. The remaining plant material is eventually broken
down by detritivores recycling those nutrients. Farmers often replenish the removed nutrients by adding fertilizer to the soil. The removal of a large number of primary producers from an ecosystem will cause some disruption to nutrient cycles.

The dynamics of an ecosystem is also disturbed when something is added to the
ecosystem which was not there previously. Often this is a man made phenomenon
when synthetic toxins and chemicals find their way into an ecosystem. Examples of toxins entering the environment are pesticides such as DDT or industrial chemicals such as PCBs. These chemicals enter the food chain and as they are passed through the different trophic levels they often increase in concentration. This phenomenon is called biological magnification. Some of these chemicals are
metabolized by organisms and the waste products are excreted from the body.
Others are stored in fatty tissue and retained in the consumer. Organisms higher
up the food chain eat a larger quantity of the organisms from lower trophic levels
and therefore consume more of the chemicals when they are stored in the body
tissues. Concentrations in an organism may reach levels where they become
disruptive to the life cycle. This was the case in the 1950s with the pesticide DDT.
High concentrations of the chemical interfered with calcium deposition in eggshells resulting in weak shells and many birds were unable to incubate their eggs. Magnification of industrial chemicals such as PCBs in biological organisms is a more recent discovery. These toxins are now found in a wide range of organisms ranging from Orca whales in the Pacific Ocean to herring gull eggs in the Great Lakes. Current research indicates that these toxins disrupt the endocrine system of animals including humans. The long term effects are not yet known. Many of these synthetic molecules cannot be broken down by microorganisms and so they persist in the ecosystem for years or even decades.
Date: 2016-01-03; view: 3812
| <== previous page | | | next page ==> |
| Construction of ecosystem structures | | | Conclusions and outlook |